EFFECT OF ANTI-MOLD AND MYCOTOXIN BINDER ON CORN QUALITY AND BROILER PERFORMANCE

Article Highlights:
- The anti-mold used effectively maintained low aflatoxin levels in 13% moisture corn.
- Synthetic mold inhibitors effectively decreased aflatoxin levels in corn during storage and maintained some nutritional quality
- Mycotoxin binder supplementation did not improve broiler
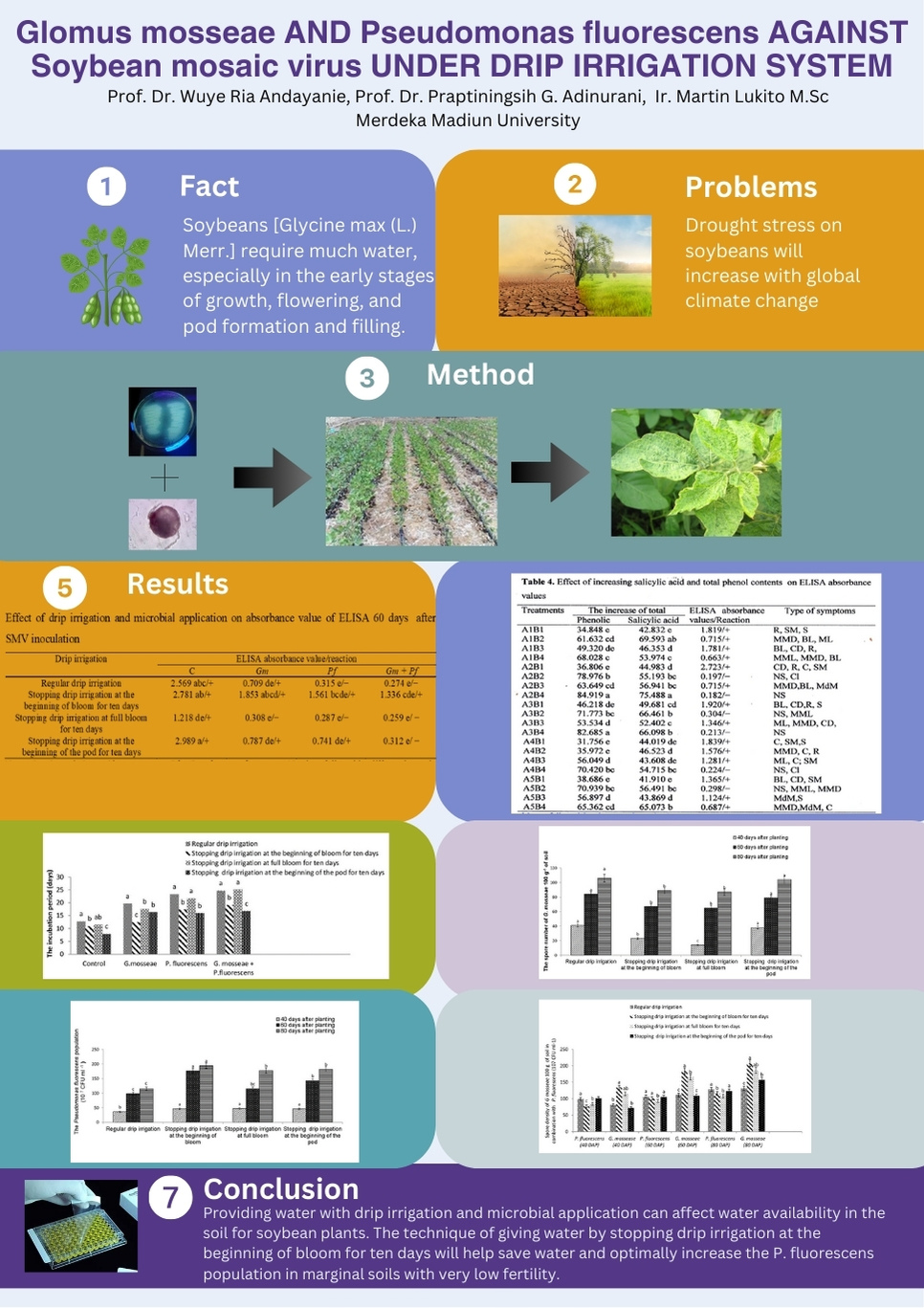
- Broiler performance declined as aflatoxin concentrations increased
Abstract:
The quality of animal feed is determined by high-quality ingredients and appropriate feed additives. This study aimed to assess: 1) the nutrient and aflatoxin total (AT) content of corn treated with an anti-mold (A) and 2) broiler performance fed with aflatoxin B1 (AFB1)-contaminated diets supplemented with a mycotoxin binder (MB). Two experiments were carried out to achieve the objectives. Experiment 1 was set up with a 2 x 2 Factorial Completely Randomized Design (FCRD) with two factors: moisture content (MC at 13 and 15%) and anti-mold (A, -/+). Meanwhile, Experiment 2 was set up with a 3 x 2 Factorial Completely Randomized Design with two factors: the AFB1 (< 100, 165, 222 µg/kg) and MB (-/ +). The MC and A interaction was significant (P < 0.01) on the aflatoxin total of corn throughout the 2-month assay. The utilization of the anti-mold in afla-corn with different moisture levels did not influence (P > 0.05) corn’s nutrient content. The MC x A interaction was significant (P < 0.05) in the valine and glycine content of the stored corn. In the second experiment, no interaction of AFB1 x MB (P > 0.05) was observed in the bird’s performance during the study. The AFB1 Concentration (AC) of corn decreased significantly (P < 0.05 to 0.001) in feed intake, body weight gain, and feed efficiency of birds. Our study concluded that the anti-mold effectively maintained low aflatoxin levels in 13% moisture corn. Also, the anti-mold did not affect the nutrient profile of corn during storage. Our study also showed that mycotoxin binder supplementation did not improve broiler performance and broiler performance declined as aflatoxin concentrations increased.
Downloads
INTRODUCTION
Mycotoxins, toxic compounds produced by pathogenic fungi, can infect animal feed and cause various negative effects on livestock. Aflatoxin, Ochratoxin, Zearalenone, and Fumonisin are examples of mycotoxins. Aflatoxin was classified into two strains, namely large (L) and small (S) strains, where the most dangerous strains were the small strains(Norlia et al., 2019)(Almatakeez, 2020). According to Mohammed et al. (2021), the L-strain Aflatoxin produced sclerotia with a size of > 400 µm, whereas the S-strain had a sclerotia with a size of < 400 µm.
Grains such as corn and peanuts are favorable media for the growth of Aspergillus spp. The presence of Aspergillus flavus in corn could be identified by the bright greeny-yellow fluorescence and black light test(Stack & Carlson, 2003). The growth of Aspergillus spp. in corn kernels depends on several factors, such as water activity, pH, and relative humidity ((Shehu & Bello, 2011);(Norlia et al., 2019)). According to Shehu and Bello (2011), the favorable temperature and relative humidity for Aspergillus spp. to grow well were 30 - 35 oC and 85 - 100%, respectively.
The growth of fungi involves two forms: yeast-like cells and mycelial growth. During their development, fungi need nutrients, such as carbohydrates (simple and complex sugars), proteins (for C, H, O, and N sources), and certain minerals(Liu et al., 2016);(Barzee et al., 2021). All nutrients are taken from the substrates on which the fungi are growing. Grains, such as corn, infested by fungi, such as Aspergillus spp., may experience a decrease in nutrient components due to the utilization of corn’s chemical components by fungi. According to(Liu et al., 2016), saccharides and proline are utilized by Aspergillus flavus for its mycelium propagation and the making of Aflatoxin B1.
The presence of aflatoxin in corn not only deteriorates corn quality but also triggers diseases and death in animals when being consumed. Aflatoxins are mutagenic, teratogenic, and carcinogenic compounds(Okechukwu et al., 2023). The amount of aflatoxin in the diet, the type of birds, and age of bird influence the harmfulness level of aflatoxin. Ducks, turkeys, and chickens are resistant to aflatoxicosis, with ducks being the most resistant species ((Wu et al., 2021);(Murcia & Diaz, 2020)). According to(Diaz & Murcia, 2019), ducks produce the highest Aflatoxin B1-dihydrodiol, causing the acute toxic effect of Aflatoxin B1.
Several efforts to control the growth and spread of toxin-producing fungi are by drying the corn immediately after harvest, by storing the corn on top of the pallets in a storage room, by using clean feed materials, and by using fungal inhibitor agents, such as charcoal and organic acids. Charcoal as an inhibitor agent works by binding to mycotoxins and excreting them through chicken excreta, thus reducing the amount of toxin absorbed in the body.
The application of certain commercial fungal inhibitors in feed succeeded in suppressing the A. flavus development but changed certain amino acid profiles in corn(Elsamra et al., 2012). Supplementation of 0.045% Mintai Feed Anti-mold effectively protected the nutritional characteristics of low-moist corn(Nalle et al., 2022).
Regarding the use of mycotoxin binder,(Nalle et al., 2021)proved that Mycosorb, as a toxin binder product, did not augment the productivity of birds given a low-dose AFB1 diet. On the contrary,(Fernandes et al., 2022)claimed that commercial toxin binders effectively increase the feed efficiency of birds that received aflatoxin diets. This indicated a contradictory result in terms of using mycotoxin binders in feed.
Feed security is still a worldwide issue, so it is important to intensively evaluate the strategy to maintain corn quality, especially using mold inhibitors (synthetic or natural). In Ethiopia, for example, a study found that 94% of poultry feed samples were contaminated with aflatoxins with a range of levels of 18 µg/kg to 190.18 µg/ kg, exceeding the FDA’s regulatory limit of 20 µg/ kg in 72.75% of samples(Kassaw et al., 2022). In Northern Pakistan, 92.5% of poultry feed samples were found positive for aflatoxins, with grower feeds exhibiting the highest contamination levels(, 2022). These findings highlight the widespread aflatoxin contamination in broiler feed across different regions. The economic impact is considerable, as aflatoxins can impair poultry health, leading to reduced growth rates, lower feed conversion efficiency, and increased mortality, thereby affecting overall productivity and profitability in the poultry industry. Aflatoxins in poultry products pose a risk to human health, potentially leading to aflatoxicosis upon consumption. Therefore, monitoring and controlling aflatoxin levels in poultry feed are crucial to mitigate these adverse effects. Considering the above problems, two experiments were conducted. In the first experiment, mold inhibitors were applied to corn with different moisture content (13% and 15%). Meanwhile, the mycotoxin binder was added to the Aflatoxin
B1 diets in the second experiment.
MATERIALS AND METHODS
Experiment I: Trial on Mold Inhibitor
Primary Materials
The primary materials of this experiment were shelled corn and anti-mold. The shelled corn used complies with the quality requirements of corn as feed ((Board, 2013)), as presented inTable 1andTable 2.
Moisture level | Whole seed | Broken seed | Moldy seed | Aflatoxin total (µg/kg) |
|---|---|---|---|---|
……………………………………%................................................. | ||||
13 | 91.98 | 2.09 | 4.85 | 29.5 |
15 | 89.90 | 2.20 | 5.00 | 52.4 |
No | Parameter | Unit | Requirements | |
|---|---|---|---|---|
Grade I | Grade II | |||
1 | Moisture Level (max) | |||
Alayande L, Bawa GS, Rano NB, Ogundipe SO. 2023. Response of broiler chickens fed with aflatoxin maize-based diets supplemented with various levels of mycotoxin binder. Slovak J Anim Sci 56(1):17-29. DOI: 10.36547/sjas.822 DOI: https://doi.org/10.36547/sjas.822
Almatakeez A. 2020. Introducing an indigenous non-toxigenic Aspergillus flavus strain isolated from Iraqi corn grains as a bio-control agent to reduce aflatoxin contamination in corn grains. [Dissertation]. Fayetteville (US): University of Arkansas.
Al-Shawabkeh K, Herzallah S, Al-Fataftah A, Zakaria H. 2009. Effect of Aflatoxin B1 contaminated feed on broiler chickens performance and meat content of conjugated linoleic acid. Jordan J Agr Sci 5(3):314-23.
Amari T, Ghnaya T, Abdelly C. 2017. Nickel, cadmium and lead phytotoxicity and potential of halophytic plants in heavy metal extraction. South Africa J Botany 111:99-110. DOI: 10.1016/j.sajb.2017.03.011 DOI: https://doi.org/10.1016/j.sajb.2017.03.011
[AOAC] Association of Official Analytical Chemists International. 2005. Official methods of analysis of AOAC International. 18th Edition. Arlington (US): Association of Official Analytical Chemists.
Barzee TJ, Caob L, Panb Z, Zhang R. 2021. Fungi for future foods. J Future Foods 1(1):25-37. DOI: 10.1016/j.jfutfo.2021.09.002 DOI: https://doi.org/10.1016/j.jfutfo.2021.09.002
Basalan M, Gungor T, Aydogan I, Hismiogullari SE, Erat S, Erdem E. 2006. Effects of feeding mycotoxin binder (HSCAS) at later ages on gastrointestinal environment and metabolism in broilers. Arc Zootech 9:1-9.
Ceci A, Massimi L, Spinelli V, Canepari S. 2020. Fungi and arsenic: Tolerance and bioaccumulation by soil aprotrophic species. App Sci 10(9):3218. DOI: 10.3390/app10093218 DOI: https://doi.org/10.3390/app10093218
Diaz GJ, Murcia HW. 2019. An unusually high production of hepatic Aflatoxin B1-dihydrodiol, the possible explanation for the high susceptibility of ducks to aflatoxin B1. Sci Rep 9(1):8010. DOI: 10.1038/s41598-019-44515-6 DOI: https://doi.org/10.1038/s41598-019-44515-6
Elsamra IA, Shama SM, Hamza AS, Youssef NH, Youssef MS, Alabd SM. 2012. Effect of treatment with mold inhibitors on plant growth of corn and some nutritional components of stored grains, infected with A. flavus and F. verticilloides. eSci J Plant Pathol 01:06-13. DOI: 10.33687/phytopath.001.01.0010 DOI: https://doi.org/10.33687/phytopath.001.01.0010
EU Monitor. 2022. Directive 2002/32/EC on undesirable substances in animal feed. European parliament and of the council.
Fernandes JIM, Baldo JS, Ferreira ACP, Schuroff JS, Reuter AH, Salinas BCD. 2022. Effect of adsorbents on diets with corn contaminated by mycotoxins on the productive performance and health of broilers. Act Sci Anim Sci 44:e53575. DOI: 10.4025/actascianimsci.v44i1.53575 DOI: https://doi.org/10.4025/actascianimsci.v44i1.53575
Gerlach, RW, Dobb DE, Raab GA, Nocerino JM. 2002. Gy sampling theory in environmental studies. 1. Assessing soil splitting protocol. J Chemometrics 16:321-8. DOI: 10.1016/S0003-2670(03)00568-3 DOI: https://doi.org/10.1002/cem.705
Hell K, Roth A. 2019. Aflatoxins and nutrition. [Internet]. Accessed on 3 August 2024. Available
from https://www.giz.de/de/downloads/giz2019_en_aflatoxins-and-nutrition.pdf
Indonesian National Standard Board 2013. Corn as Feed. ISSN 1:4483-2013. Jakarta (ID):INSB. [in Indonesian].
Kassaw T, Megerssa YC, Woldemariyam FT. 2022. Occurrence of aflatoxins in poultry feed in selected chicken rearing villages of Bishoftu Ethiopia. Vet Med Res Rep 13:277-86. DOI: 10.2147/vmrr.s384148 DOI: https://doi.org/10.2147/VMRR.S384148
Kolossova A, Stroka J, Breidbach A, Kroeger K, Ambrosio M, Bouten K, Ulberth F. 2011. Evaluation of the effect of mycotoxin binders in animal feed on the analytical performance of standardised methods for the determination of mycotoxins in feed. European Commission, Joint Research Centre, Institute for Reference Materials and Measurement. Luxembourg (LU): Publications Office of the European Union.
Liu J, Sun L, Zhang N, Zhang J,Guo J, Li C, Rajput SA, Qi D. 2016. Effects of nutrients in substrates of different grains on Aflatoxin B1 production by Aspergillus flavus. BioMed Res Int 2016:7232858. DOI: 10.1155/2016/7232858 DOI: https://doi.org/10.1155/2016/7232858
Mendoza DGE, Purnamasari L, Olarve JP, dela Cruz JF. 2022. Effects of toxin binder supplementation via drinking water on the growth performance of broiler chickens. Anim Prod 24(3):142-9. DOI: 10.20884/1.jap.2022.24.3.168 DOI: https://doi.org/10.20884/1.jap.2022.24.3.168
Mijena D, Ijara F. 2024. Livestock feed anti-nutritional components: A review. J Nut Food Process 7(6). DOI:10.31579/2637-8914/231
Mohammed A, Faustinelli PC, Chala A, Dejene M, Fininsa C, Ayalew A, …, Arias RS. 2021. Genetic fingerprinting and aflatoxin production of Aspergillus section Flavi associated with groundnut in eastern Ethiopia. BMC Microbiol 21(1):239. DOI: 10.1186/s12866-021-02290-3 DOI: https://doi.org/10.1186/s12866-021-02290-3
Monson MS, Coulombe RA, Reed KM. 2015. Aflatoxicosis: Lessons from toxicity and responses to Aflatoxin B1 in poultry. Agr 5(3):742-77. DOI: 10.3390/agriculture5030742 DOI: https://doi.org/10.3390/agriculture5030742
Mulyati AH, Sutanto, Warnasih S Herlina E. 2015. Determination of the aflatoxin levels in corn (Zea mays, L.) during storage process. J Phys Conf Ser 1882(1):012115. DOI: 10.1088/1742-6596/1882/1/012115 DOI: https://doi.org/10.1088/1742-6596/1882/1/012115
Murcia H, Diaz GJ. 2020. Dealing with Aflatoxin B1 dihydrodiol acute effects: Impact of Aflatoxin B1-aldehyde reductase enzyme activity in poultry species tolerant to AFB1 toxic effects. PLoS One 15(6):e0235061. DOI: 10.1371/journal.pone.0235061 DOI: https://doi.org/10.1371/journal.pone.0235061
Nalle, CL, Supit MAJ, Akbar AM, So’o A, Langodai E. 2022. Physical and chemical qualities of corn with different moisture levels supplemented with mold inhibitor. BIOTROPIA 29(3):234-43. DOI: 10.11598/btb.2022.29.3.1705 DOI: https://doi.org/10.11598/btb.2022.29.3.1705
Nalle CL, Supit MAJ, Angi AH, Yuliani NS. 2021. The performance, nutrient digestibility, Aflatoxin B1 residue, and histopathological changes of broilers exposed to dietary mycosorb. Trop Anim Sci J 44(2):160-72. DOI: 10.5398/tasj.2021.44.2.160 DOI: https://doi.org/10.5398/tasj.2021.44.2.160
Naveed IT, Haleem KS, Bano N, Ghazanfar S, Adetunji CO, Saleem MH, …, Paray BA. 2022. Quantitative estimation of aflatoxin level in poultry feed in selected poultry farms. BioMed Res Int 2022:1-7. DOI: 10.1155/2022/5397561 DOI: https://doi.org/10.1155/2022/5397561
Nazarizadeh H, Pourreza J. 2019. Evaluation of three mycotoxin binders to prevent the adverse effects of aflatoxin B1 in growing broilers. J Appl Anim Res 47:135-9. DOI: 10.1080/09712119.2019.1584106 DOI: https://doi.org/10.1080/09712119.2019.1584106
Norlia M, Jinap S, Nor-Khaizura MAR, Radu S, Samsudin NIP, Azri FA. 2019. Aspergillus section Flavi and Aflatoxins: Occurrence, detection, and identification in raw peanuts and peanut-based products along the supply chain. Front Microbiol 10:2602. DOI: 10.3389/fmicb.2019.02602 DOI: https://doi.org/10.3389/fmicb.2019.02602
Okechukwu V, Adelusi OA, Kappo AP, Njobeh PB. 2023. Aflatoxins: Occurrence, biosynthesis, mechanism of action and effects, conventional/emerging detection techniques. Food Chem 436(2):137775. DOI: 10.1016/j.foodchem.2023.137775 DOI: https://doi.org/10.1016/j.foodchem.2023.137775
Payne GA, Hagler WM. 1983. Effect of specific amino acids on growth and aflatoxin production by Aspergillus parasiticus and Aspergillus flavus in defined media. Appl Environ Microbiol 46(4):805-12. DOI: 10.1128/aem.46.4.805-812.1983 DOI: https://doi.org/10.1128/aem.46.4.805-812.1983
Popescu RG, Rădulescu AL, Georgescu SE, Dinischiotu A. 2022. Aflatoxins in feed: Types, metabolism, health consequences in swine and mitigation strategies. Toxins 14(12):853. DOI: 10.3390/toxins14120853 DOI: https://doi.org/10.3390/toxins14120853
Prahara GW, Humaer A, Yusiati LM, Hanim C, Kurniawati A, Sumantri I, …, Al Ana M. 2023. Effect of phyllosilicates as toxin binder on productivity, intestinal morphology, and liver toxicity in broiler fed AFB1 contaminated feed. Bul Anim Sci 47(2):82-90. DOI: 10.21059/buletinpeternak.v47i2.81410 DOI: https://doi.org/10.21059/buletinpeternak.v47i2.81410
Prawira I, Rukmi I, Wijanarka. 2015. Production of Aspergillus flavus Pam-25 protease enzyme with variations in pH and incubation time. Biol J 4(2):10-6.
SAS Institute. SAS/STAT® User's Guide: Statistics. (SAS OnDemand for Academics- Free Online Software). Cary (US): SAS Institute Inc.
Shabeer S, Asad S, Jamal A, Ali A. 2022. Aflatoxin contamination, its impact and management strategies: An updated review. Toxins 14(5):307. DOI: 10.3390/toxins14050307 DOI: https://doi.org/10.3390/toxins14050307
Shehu K, Bello MT. 2011. Effect of environmental factors on the growth of Aspergillus species associated with stored millet grains in Sokoto. Nig J Basic App Sci 19(2):218-23.
Stack J, Carlson M. 2003. NF571 Aspergillus flavus and aflatoxins in corn. University of Nebraska-Lincoln: Historical Materials, Extension. 43. [Internet]. Accessed on 15 June 2023. Available from: https://digitalcommons.unl.edu/extensionhist/43.
Wu G. 2013. Amino acids: Biochemistry and nutrition. Boca Raton (US): CRC Press, Taylor & Francis Group.
Wu K, Liu M, Wang H, Rajput SA, Qi D, Wang S. 2021. The mechanism underlying the extreme sensitivity of duck to Aflatoxin B1. Oxid Med Cell Long 2021(1):1-8. DOI: 10.1155/2021/9996503 DOI: https://doi.org/10.1155/2021/9996503
Xu R, Kiarie EG, Yiannikouris A, Sun L, Karrow NA. 2022. Nutritional impact of mycotoxins in food animal production and strategies for mitigation. J Anim Sci Biotech 13:69. DOI: 10.1186/s40104-022-00714-2 DOI: https://doi.org/10.1186/s40104-022-00714-2
Yun J, Lee DG. 2016. A novel fungal killing mechanism of propionic acid. FEMS Yeast Res 16:1-7. DOI: 10.1093/femsyr/fow089 DOI: https://doi.org/10.1093/femsyr/fow089
Zabiulla I, Malathu V, Swamy HVLN, Naik J, Pineda L, Han Y. 2021. The efficacy of a smectite-based mycotoxin binder in reducing Aflatoxin B1 toxicity on performance, health and histopathology of broiler chickens. Toxins 13(12):856. DOI: 10.3390/toxins13120856 DOI: https://doi.org/10.3390/toxins13120856
Zhu F, Zhu L, Xu J, Wang Y, Wang Y. 2023. Effects of moldy corn on the performance, antioxidant capacity, immune function, metabolism and residues of mycotoxins in eggs, muscle, and edible viscera of laying hens. Poult Sci 102:102502. DOI: 10.1016/j.psj.2023.102502 DOI: https://doi.org/10.1016/j.psj.2023.102502
Zwolak I. 2020. The role of Selenium in Arsenic and Cadmium toxicity: An updated review of scientific literature. Bio Trace Elem Res 193:44-63. DOI: 10.1007/s12011-019-01691-w DOI: https://doi.org/10.1007/s12011-019-01691-w
Copyright (c) 2025 CATOOTJIE LUSJE NALLE, Mr. Supit, Mr. Jermias, Drh. Kusumaningrum, Miss Ariska, Miss. Melami

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree with the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work 1 year after publication simultaneously licensed under a Creative Commons attribution-noncommerical-noderivates 4.0 International License that allows others to share, copy and redistribute the work in any medium or format, but only where the use is for non-commercial purposes and an acknowledgement of the work's authorship and initial publication in this journal is mentioned.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).