ANTIBACTERIAL AND DIASTASE ENZYME ACTIVITIES OF HONEY Apis mellifera FROM INDONESIA
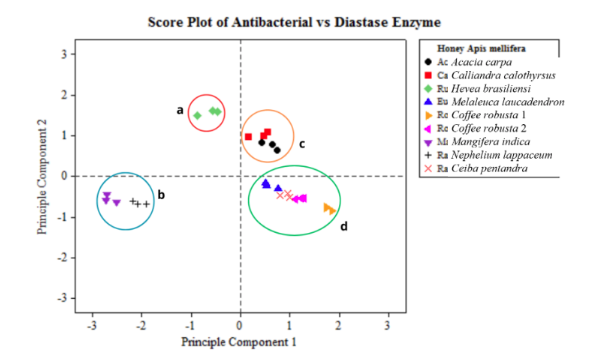
The quality of monofloral honey from Apis mellifera as an antibacterial can be influenced by the activity of the diastase enzyme and the secondary metabolites from the nectar source. Therefore, this study aimed to compare the activity of the diastase enzyme and the effectiveness of monofloral honey derived from Apis mellifera bees as a natural antibacterial agent against Staphylococcus aureus and Escherichia coli. Sampling for monofloral honey was carried out from nine different nectar sources, namely Acacia carpa, Calliandra calothyrsus, Nephelium lappaceum, Melaleuca laucadendron, Ceiba pentandra, Mangifera indica, Coffea robusta 1, Coffea robusta 2, and Hevea brasiliensisensi. Furthermore, diastase content was determined using UV-Vis spectrophotometry and the bacterial inhibition zone using the disc diffusion method. The principal component analysis (PCA) was used to analyze the clustering of diastase enzyme and antibacterial activity. The results showed that the highest diastase activity in monofloral honey was Mangifera indica, Nephelium lappaceum, and Coffea robusta 2 at 20.00 DN. This was followed by Nephelium lappaceum, Ceiba pentandra, and Hevea brasiliensisensisensi at 10.00 DN, Acacia carpa at 6.67 DN, Coffea robusta 1 at 5.00 DN, and Calliandra calothyrsus 4.00 DN. The clear zones for Staphylococcus aureus on Coffea robusta 2, Acacia carpa, Nephelium lappaceum, Coffea robusta 1, Ceiba pentandra, Hevea brasiliensisensisensi, Nephelium lappaceum, Calliandra calothyrsus, and Mangifera indica were 19.47, 18.53, 17.73, 17.03, 16.12, 16.10, 16.03, 15.73, and 14.73 mm, respectively. Additionally, the clear zones for Escherichia coli on Ceiba pentandra, Coffea robusta 2, Acacia carpa, Coffea robusta 1, Melaleuca laucadendron, Mangifera indica, Hevea brasiliensisensisensi, Calliandra calothyrsus, and Nephelium lappaceum were 27.93, 26.13, 24.60, 24.53; 24.53, 24.07, 21.90, 21.60, and 21.53 mm, respectively. In conclusion, clustering analysis was conducted based on nectar sources to evaluate antibacterial and diastase activity. The clusters identified are cluster 1 consisting of Hevea brasiliensisensi, cluster 2 including Mangifera indica, and Nephelium lappaceum. Others are cluster 3 consisting of Acacia carpa and Calliandra calothyrsus, and clustergroup 4 including Nephelium lappaceum, Ceiba pentandra, Coffea robusta 1, and Coffea robusta 2. Therefore, it was necessary to carry out antibacterial testing of other bacteria, specifically Salmonella typhi, and determine the minimum inhibitory concentration (MIC) of honey with the best antibacterial activity in various concentration variations.
Downloads
Almasaudi S. 2021. The antibacterial activity of honey. Saudi J Biol Sci 28(2021): 2188-2196. DOI: https://doi.org/10.1016/j.sjbs.2020.10.017
Andayani RP. 2020. Honey as a complementary therapy to overcome diarrhea in children under five. Perintis’s Health J 7(1): 64-68. DOI: https://doi.org/10.33653/jkp.v7i1.393
Arumsari A, Herawati D, Afrizal M. 2019. Antibacterial activity test of several types of honey against Pseudomonas aeruginosa and Staphylococcus aureus by agar diffusion method. JIF Framasyifa 2(1): 26-32.
Borgen A, Ferreira C, Saavedra MJ, Simoes M. 2013. Antibacterial activity and mode of action of ferulic and gallic acid against pathogenic bacteria. Microb Drug Resist 19(4): 256-265. DOI: https://doi.org/10.1089/mdr.2012.0244
Chassagne F, Samarakoon T, Porras G, Lylees JT, Dettweuler M, Maequez L, Salam AM, Habih S, Farrokhi DR, Quave CL. 2021. A systematic review of plant with antibacterial activities: a taxonomic and phylogenetic perspective. Front Pharmacol 11(2021): 1-29. DOI: https://doi.org/10.3389/fphar.2020.586548
[CLSI] Clinical Laboratory Standart Institute. 2013. Performance Standard for Antimicrobial Susceptibility Testing. USA(US): Twentieth Information Supplement.
Collins W, Lowen N, Blake DJ. 2019. Caffeic acid esters are effective bactericidal compounds. Biomolecules 9(8): 1-13. DOI: https://doi.org/10.3390/biom9080312
Das A, Datta S, Mukhrejee S, Bose S, Ghosh S, Dhar P. 2015. Evaluation of antioxidative, antibacterial, and probiotic growth stimulatory activities of Sesamum indicum honey containing phenolic compounds and lignin. LWT-Food Sci Tech 61(1): 244-250. DOI: https://doi.org/10.1016/j.lwt.2014.11.044
Evahelda E, Pratama F, Malahayati N, Santoso. 2017. Physical and chemical properties of honey from rubber tree nectar in Central Bangka District, Indonesia. Agritech 37(4): 363-368. DOI: https://doi.org/10.22146/agritech.16424
Ghani ZDFA, Husin JM, Rashid AHA, Shaari K, Chik Z. 2016. Biochemical studies of Piper betle L extract on the obese treated animal using 1H-NMR-based metabolomic approach of blood serum samples. J Ethnopharmacol. 194(2016): 1-25. DOI: https://doi.org/10.1016/j.jep.2016.10.022
Gonzalez-Ceballos L, Fernandez-Muino MA, Oses SM, Sancho MT, Ibeas S, Ruiz JAR, Vallejos S. 2021. Polymer film as a starch azure container for the easy diastase activity determination in honey. Food Chem 335(2021): 1-5. DOI: https://doi.org/10.1016/j.foodchem.2021.129629
Gorniak I, Bartoszewski R, Kroliczewski J. 2019. A comprehensive review of antimicrobial activities of plant flavonoids. Phytocem Rev 18(1): 241-272. DOI: https://doi.org/10.1007/s11101-018-9591-z
Gunes N, Aydin L, Beleni D, Hranitz JM, Mengilig S, Selova S (2017) Stress responses of honey bees to organic acid and essential oil treatment against varroa mites. J of Agricultural Res 56(2): 175-181. DOI: https://doi.org/10.1080/00218839.2017.1291229
Hanifa F, Purwaningrum R, Mustofa F, Zulfan. 2020. Effectiveness of pure honey and propolis against milk contaminating bacteria that cause foodborne disease in packaged milk. J Health 9(1): 47-52. DOI: https://doi.org/10.35816/jiskh.v11i1.217
Harjo SST, Radiati LE, Rosyidi D. 2015. Comparison of rubber honey and rambutan honey based on water content, diastase enzyme activity, and hydroxymethylfurfural (HMF). J Production Sci Tech Livestock Products 10(1): 18-21. DOI: https://doi.org/10.21776/ub.jitek.2015.010.01.3
Hasan AEZ, Herawati H, Purnomo, Amalia L. 2020. Physicochemistry of multiflora honey from Riau and its potential as an antibacterial Escherichia coli and Staphylococcus aureus. Chem. Prog 13(2): 81-90. DOI: https://doi.org/10.35799/cp.13.2.2020.31594
Ichsan DS, Nurdin I, Hafidzah TS, Putri BS, Aurene SV. 2022. Fake honey detection and honey quality with diastase enzyme test. Poltekita: J Health Sci 16(3): 278-283. DOI: https://doi.org/10.33860/jik.v16i3.1685
Kaligis CJ, Nangoy E, Mambo CD. 2020. Test the antibacterial effect of forest honey and black honey against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa bacteria. eBiomedik 8(1): 112-119.
[Ministry of Health of the Republic of Indonesia] Ministry of Health of the Republic of Indonesia. 2020. Indonesian Health Profile. Jakarta(ID): Directorate General of Disease Control and Environmental Health.
Muziburrahman M, Husada D, Utomo B. 2022. Identification of bacteria causing diarrhea in under-five children using culture method in Bima, Indonesia. Periodic Epidemiol J 10(1): 95-102. DOI: https://doi.org/10.20473/jbe.V10I12022.95-102
Liu JR, Ye YL, Lin TY, Wang YW, Peng CC. 2013. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honey in Taiwan. Food Chem 139(1-4): 938-943. DOI: https://doi.org/10.1016/j.foodchem.2013.02.015
Molan P, Rhodes T. 2015. Honey: a biologic wound dressing. J Wounds 27(6): 141-150.
Nasharuddin MA, Sunaryo, Puspitarini OR. 2022. Analysis of the quality of acacia honey, rubber, and randu produced by PT Kembang Joyo Sriwijaya. Dinamika Rekasatwa J 5(2): 169-173.
Nayik GA, Nanda V. 2015. Physico-chemical, enzymatic, mineral, and colour characterization of three different varieties of honey from khasmir valley of India with a multivariate approach. J Food Nutr Sci 65(2): 101-108. DOI: https://doi.org/10.1515/pjfns-2015-0022
Obistioui D, Covan I, Tirzui E, Herman V, Megrea M, Cucerzan A, Naescu AG, Cizma AL, Nichita I, Hulea A, Radoluv I, Alexa E. 2021. Phytochemical profile and microbiological activity of some plants belonging to the fabaceae family. Antibiotic 10(62): 1-20. DOI: https://doi.org/10.3390/antibiotics10060662
Panjaitan R, Darmawati S, Prastiyanto M. 2018. Antibacterial activity of honey against multi-drug resistant Salmonella typhi and methicillin-resistant Staphylococcus aureus. Unimus FMIPA Education National Seminar on Science and Technology 2018. Muhammadiyah University of Semarang, Semarang [Indonesia].
Rohman A. 2017. Physico-chemical properties and biological activities of rambutan (Naphelium lappaceum L) fruit. Res J Phytochem 11(2): 66-73. DOI: https://doi.org/10.3923/rjphyto.2017.66.73
Shi C, Zhang X, Sun Y, Wang M, Song Y, Zheng Z, Chen Y, Liu X, Jia Z, Dong R, Cui LU, Xia X. 2016. Antimicrobial activity of ferulic acid against Cronobakter sakazakii and possible mechanism of action. Foodborne Pathog Dis 13(4): 196-204. DOI: https://doi.org/10.1089/fpd.2015.1992
Singh I, Singh S. 2018. Honey moisture reduction and quality. J Food Sci Technol 55(10): 3861-3871. DOI: https://doi.org/10.1007/s13197-018-3341-5
Soares S, Grazina L, Mafra I, Costa J, Pinto MA, Duc HP, Oliveira MBPP, Amaral JS. 2018. Novel diagnostic tools for Asian (Apis cerana) and European (Apis mellifera) honey authentication. Food Res Int 105: 686-693. DOI: https://doi.org/10.1016/j.foodres.2017.11.081
[SNI] Indonesian National Standard 8664:2018 (2018) Standardization of Honey Quality. Jakarta(ID): National Standardization Body.
Sukmawati, Noor A, Firdaus. 2015. Analysis of the quality of Mallawa honey based on physicochemical parameters. Ind J Chem Res 2015(3): 259-262.
Swari NKI, Wirawan R, Qomariyah N, Al Hadi K (2019) Analysis of the water content in honey using a combination of capacitance and refractive index methods. KONSTAN 4(1): 1-10. DOI: https://doi.org/10.20414/konstan.v4i1.21
Tulandi SM. 2019. The effect of storage temperature on the quality of honey. SANITAS 10(1): 59-71. DOI: https://doi.org/10.36525/sanitas.2019.6
Wadi MA. 2022. In vitro antibacterial activity of different honey samples against clinical isolates. Biomes Res Int 2022: 1-8. DOI: https://doi.org/10.1155/2022/1560050
World Health Organization (WHO) [Internet]. 2018. Diarrhoeal Disease. [updated 2017 May 2; cited 2023 Jan 22]. Available from: https:// www.who.int/en/news-room/fact-sheets/detail/ diarrhoeal-disease.
Yuliati. 2017. Test the effectiveness of honey solution as an antibacterial agent in the growth of Staphylococcus aureus and Pseudomonas aeruginosae using the disk diffusion method. Med Professional J 11(1): 7-15. DOI: https://doi.org/10.33533/jpm.v11i1.206
Zhang YZ, Chen YF, Wu YQ, Si JJ, Zhang CP, Zheng HQ, Hu FL. 2019. Discrimination of the entomological origin of honey according to the secretions of the bee (Apis cerana or Apis mellifera). Food Res Int 116: 362-369. DOI: https://doi.org/10.1016/j.foodres.2018.08.049
Copyright (c) 2024 Rara Annisaur Rosyidah, Akhmad Endang Zainal Hasan, Dimas Andrianto

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree with the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work 1 year after publication simultaneously licensed under a Creative Commons attribution-noncommerical-noderivates 4.0 International License that allows others to share, copy and redistribute the work in any medium or format, but only where the use is for non-commercial purposes and an acknowledgement of the work's authorship and initial publication in this journal is mentioned.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).