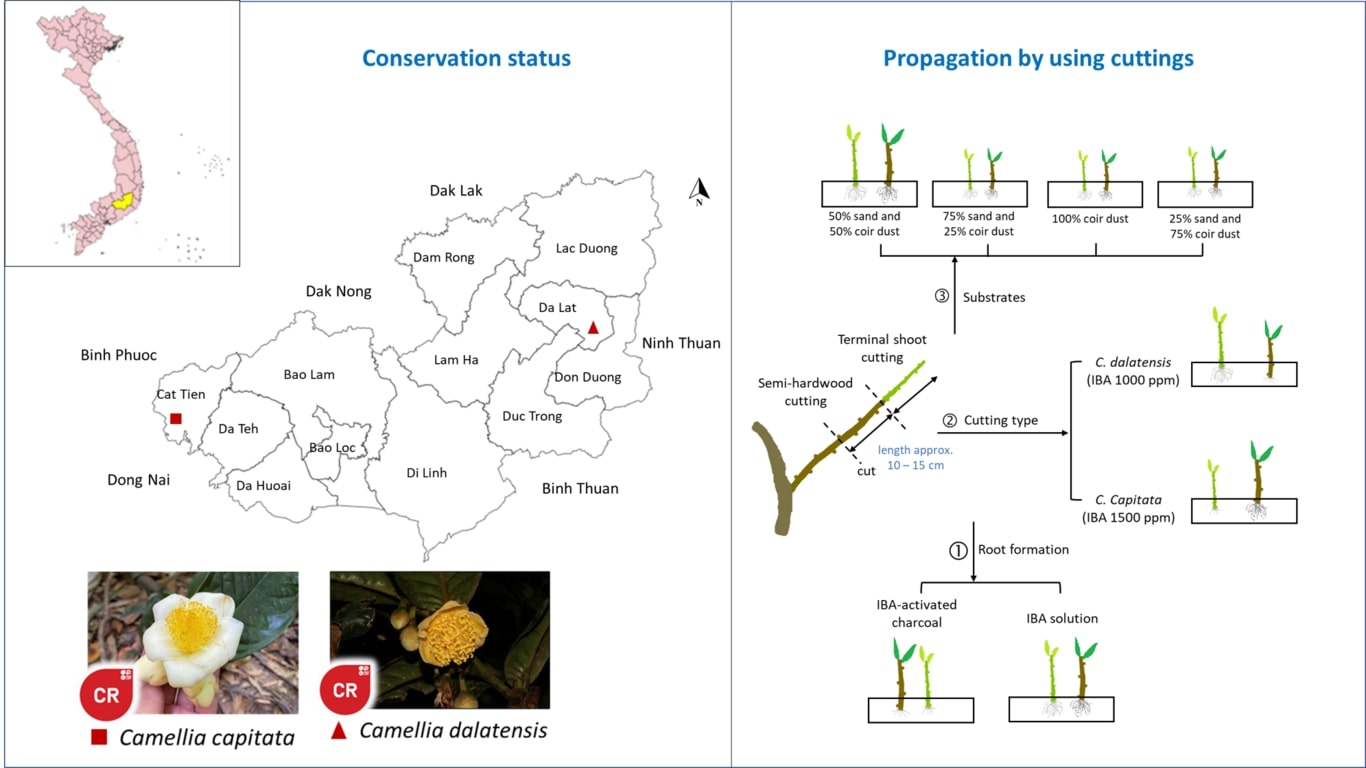
PLANT REGENERATION THROUGH PROTOCORM-LIKE BODIES DERIVED FROM STEM THIN LAYER OF Anubias barteri var. Nana Petite
Article Highlights
- Effective induction of PLBs from stem thin layer and shoot regeneration using specific BAP and auxin combinations.
- Significant role of potato extract in enhancing shoot regeneration.
- Highest shoot regeneration rate achieved with 3 mg/L BAP concentration.
Abstract
Anubias barteri var. nana Petite, a highly valued ornamental aquatic plant in the Araceae family, faces challenges in vegetative propagation due to its slow multiplication rate. This study established a robust micropropagation protocol for rapid and efficient multiplication of A. barteri var. nana Petite. Explants were subjected to thorough sterilization, and shoot induction and multiplication were optimized using varying concentrations of Benzyl adenine (BA) on Murashige and Skoog (MS) medium. Protocorm-like bodies (PLBs) were induced from transverse thin-layer explants using combinations of BAP and Naphthaleneacetic acid (NAA). PLB multiplication and shoot regeneration were achieved through a sequential culture protocol. In addition, the influence of potato extract on shoot regeneration and the role of Indole-3-butyric Acid (IBA) in root development were explored. This study demonstrated a 100% survival rate of regenerated shoots in aquariums. Ongoing research is focused on further enhancing PLB multiplication. This optimized micropropagation protocol holds promise for the large-scale production of A. barteri var. nana Petite, addressing the limitations of natural propagation.
Downloads
INTRODUCTION
Anubias barteri var. nana Petite holds significant commercial importance as a highly sought-after decorative aquatic plant within the Araceae family. This cultivated variant, renowned for its slow growth and cluster formation, typically ranging from 1 cm to 5 cm in height, has garnered popularity in both aquatic and ornamental settings(George et al., 2015).
Despite its aesthetic appeal, the natural propagation of A. barteri has proven inefficient, leading to numerous studies on micropropagation techniques, including in vitro multiplication(George et al., 2015);(Huang et al., 1994);(Kanchanapoom et al., 2012);(Rittirat et al., 2021), in vitro organogenesis(Surendra et al., 2019);(Rittirat et al., 2023), and hydroponic culture(Sholichah et al., 2020). However, these efforts have resulted in an average shoot production ranging between 2 shoots to 5 shoots from a single shoot.
To address this limitation, the utilization of protocorm-like bodies (PLBs) presents a promising approach for mass production because of their rapid formation, uniform characteristics, disease-free nature, and sustainability(Cardoso et al., 2020);(Sheelavanthmath et al., 2005). Noteworthy success has been achieved in the utilization of PLBs for the proliferation and multiplication of ornamental plants within the Araceae family. For example, Anthurium andreanum cv. CanCan exhibited 97.8% rate of PLB induction, averaging 120 PLBs for each explant over a period of 50 days in cultivation(Gantait et al., 2012);(Yu et al., 2009). Similarly, TDZ was shown effective for inducing PLBs from leaf explants of Cattleya tigrine with 17.5% induction rate, and GA3significantly increases shoot regeneration from PLBs with approximately 220 developed plantlets per culture flask(Fritsche et al., 2022).
Despite these successes, there is currently no documented report on regeneration from PLBs on Anubias barteri var. nana Petite. Given the documented success of PLBs in various plant species, this study aimed to identify the optimal concentrations of plant growth regulators and potato extract for propagating this species through shoot tips and PLBs. The development of this micropropagation protocol provides a methodical approach to augment the propagation of Anubias barteri var. nana Petite, effectively overcoming the limitations of natural propagation methods.
MATERIALS AND METHODS
Shoot Sterilization
Shoots of Anubias barteri var. nana Petite were subjected to surface sterilization using soap for 15 minutes, followed by exposure to an antifungal agent (Mancozeb, India) for 10 minutes, washed with running water, and further rinsed with 70% alcohol for 30 seconds. Subsequently, explants were soaked in a commercial bleach solution (5% sodium hypochlorite) at concentrations of 10%, 20%, 30%, and 40% (v/v) for 15 minutes, followed by a 30-minute soak with an antibiotic solution (1 mg/mL). After disinfection, 3-5 mm shoot tip explants were isolated and placed on MS (Murashige and Skoog, 1962) basal medium.
Shoot Multiplication from Shoot Tips
Shoot tips free of microbial infection were cultured in MS media supplemented with (0, 1.0, 2.0, 3.0, 4.0 mg/L) BAP for 6 weeks for shoot induction. In a subsequent experiment, a single shoot (approximately 0.8 cm in height) was transferred to MS medium supplemented with various BAP concentrations (2.0, 3.0, 4.0 mg/L) for multiplication over 4 weeks. Shoot multiplication parameters, including the number of shoots, shoot height, number of leaves per shoot, and leaf length were recorded.
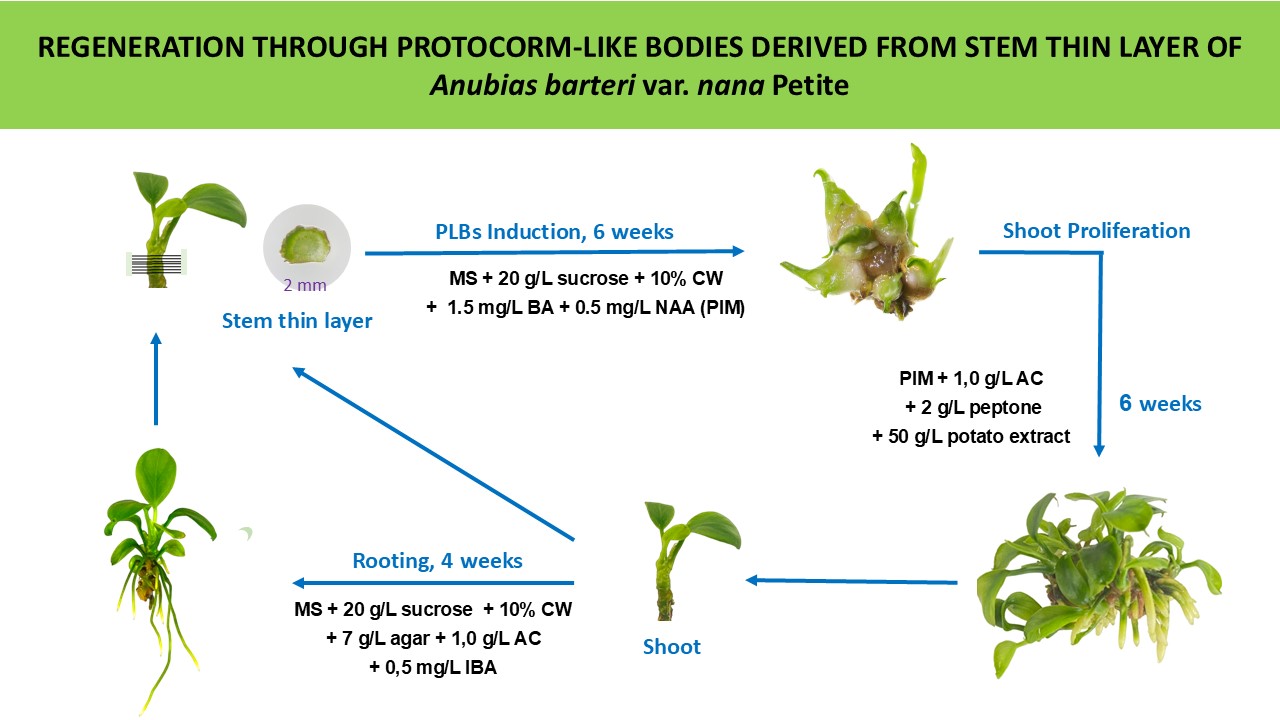
Protocorm-like Bodies (PLBs) Induction, Proliferation and Shoot Regeneration
For PLB induction, in vitro stems were separated from the cluster, leaves were removed, and the stem was cut into 1-2 mm transverse slices. These slices were placed on MS medium containing 10% (v/v) coconut water (CW), 2% (w/v) sucrose, and complemented with (0.5-3.0 mg/L) BAP along with (0.5-2.0 mg/L) NAA. The percentage of PLB induction and the number of PLBs per explant were recorded after 6 weeks of dark culturing. PLB morphology and counting were performed at 2X to 4X magnification under a stereo microscope (Olympus SZ51, Japan).
Secondary PLB Induction and Shoot Regeneration
For PLB proliferation, PLB clusters were separated into individuals and placed on MS medium containing 10% (v/v) CW, 2% (w/v) sucrose, added (0.5-1.5 mg/L) BA and (0.2-0.5 mg/L) IAA. After 3 weeks, the secondary PLB number and diameter, indicating PLB growth speed, were recorded.
To regenerate shoots from PLBs, tuber-shaped PLBs were separated and transferred to MS medium containing 10% (v/v) CW, 2% (w/v) sucrose, 1.0 g/L activated charcoal, 2 g/L peptone, and 1-3 mg/L BA and 0.5 mg/L IBA. After 3 weeks, shoot height was measured, and shoot quality was determined by leaf color, stem diameter, and leaf length.
Effect of Potato Extract on Shoot Proliferation
To assess the impact of potato extract on shoot proliferation, PLBs were cultured on MS medium complemented with 1.5 mg/L BAP and 0.5 mg/L NAA ranging from 10 g/L to 50 g/L of PE. Data on shoot number and individual shoot height were collected after the designated culture period.
Rooting
In vitro plantlets (1-1.5 cm) regenerated from PLBs were cultured on MS medium supplemented with 0.1% (w/v) activated carbon, 10% (v/v) CW, and Indole-3-Butyric Acid (IBA) at different concentrations (0.5, 1.0, 1.5, 2.0 mg/L). After 4 weeks, the number of roots per shoot, root length, shoot height, and number of leaves per shoot were recorded.
Experimental Design and Statistical Analysis
All experiments were conducted in a Completely Randomized Design (CRD) with three repetitions for each treatment, and each repetition included five jars, each containing at least one explant. The data underwent basic statistical analysis and were examined using
Ajithkumar D, Seeni S. 1998. Rapid clonal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Reports. 17: 422-6. DOI: 10.1007/s002990050418 DOI: https://doi.org/10.1007/s002990050418
Cardoso JC, Zanello CA, Chen JT. 2020. An overview of orchid protocorm-like bodies: Mass propagation, biotechnology, molecular aspects, and breeding. Int J Mol Sci 21(3):985. DOI: https://doi.org/10.3390/ijms21030985
Cui J, Liu J, Deng M, Chen J, Henny RJ. 2008. Plant regeneration through protocorm-like bodies induced from nodal explants of Syngonium podophyllum “White Butterfly.” Hortic Sci 43:2129-33. DOI: 10.21273/HORTSCI.43.7.2129 DOI: https://doi.org/10.21273/HORTSCI.43.7.2129
Fritsche Y, Deola F, da Silva DA, Holderbaum DF, Guerra MP. 2022. Cattleya tigrina (Orchidaceae) in vitro regeneration: Main factors for optimal protocorm-like body induction and multiplication, plantlet regeneration, and cytogenetic stability. S Afr J Bot 149:96-108. DOI: https://doi.org/10.1016/j.sajb.2022.05.059
Gantait S, Sinniah UR, Mandal N, Das PK. 2012. Direct induction of protocorm-like bodies from shoot tips, plantlet formation, and clonal fidelity analysis in Anthurium andreanum cv. CanCan. J Plant Growth Regul 67:257-70. DOI: 10.1007/s10725-012-9684-4 DOI: https://doi.org/10.1007/s10725-012-9684-4
George S, Joseph A, Korath A. 2015. In vitro propagation of an ornamental aquatic plant, Anubias barteri var. nana Petite. Res J Article 18:1-12.
Huang LC, Chang YH, Chang YL. 1994. Rapid in vitro multiplication of the aquatic angiosperm, Anubias barteri var. undulata. Aquat Bot 47(1):77-83. DOI: 10.1016/0304-3770(94)90050-7 DOI: https://doi.org/10.1016/0304-3770(94)90050-7
Islam MO, Akter M, Prodhan AKMAUD. 2012. Effect of potato extract on in vitro seed germination and seedling growth of local Vanda roxburgii orchid. J Bangladesh Agril Univ 9:211-5. DOI: 10.22004/ag.econ.208642 DOI: https://doi.org/10.3329/jbau.v9i2.10988
Kanchanapoom K, Chunui P, Kanchanapoom K. 2012. Micropropagation of Anubias barteri var. nana from shoot tip culture and the analysis of ploidy stability. Not Bot Horti Agrobo 40:148-51. DOI: 10.15835/nbha4027520 DOI: https://doi.org/10.15835/nbha4027520
Komal R. 2011. Effect of BAP and IAA on callus formation and plant regeneration in pointed gourd. Biotechnology, Bioinformatics and Bioengineering 1(1):59-62.
Lee YI, Lee N. 2003. Plant regeneration from protocorm-derived callus of Cypripedium formosanum. In Vitro Cellular & Developmental Biology. Plant 39:475-9. DOI: 10.1079/IVP2003450 DOI: https://doi.org/10.1079/IVP2003450
Mondal T, Aditya S, Banerjee N. 2014. In vitro axillary shoot regeneration and direct protocorm-like body induction from axenic shoot tips of Doritis pulcherrima Lindl. Plant Tissue Cult Biotechnol 23:251-61. DOI: https://doi.org/10.3329/ptcb.v23i2.17526
Rahman ARMM, Islam MO, Ichihashi S. 2004. Effects of complex organic extracts on plantlet regeneration from PLBs and plantlet growth in the Doritaenopsis orchid. Jpn Agr Res Q 38(1):55-9. DOI: 10.6090/jarq.38.55 DOI: https://doi.org/10.6090/jarq.38.55
Ram M. 2012. In vitro plant regeneration of Esmeralda clarkei Rchb.f. via protocorm explant. Afr J Biotechnol 11(54):11704-8. DOI: 10.5897/AJB12.985 DOI: https://doi.org/10.5897/AJB12.985
Rittirat S, Klaocheed S, Kalawong S, Thammasiri K. 2023. Adventitious shoot organogenesis from shoot tip explants of an ornamental aquatic plant Anubias barteri var. nana. Proceedings book: 20.
Rittirat S, Klaocheed S, Thammasiri K. 2021. Thidiazuron induces high-frequency plant regeneration from shoot tip explants of an ornamental aquatic plant, Anubias barteri var. nana. In IX International Scientific and Practical Conference on Biotechnology as an Instrument for Plant Biodiversity Conservation. 1339:273-80. DOI: https://doi.org/10.17660/ActaHortic.2022.1339.34
Rittirat S, Klaocheed S, Thammasiri K, Walam A. 2019. An efficient in vitro plantlet regeneration of an ornamental aquatic plant Anubias heterophylla through shoot tip culture. In International Symposium on Botanical Gardens and Landscapes. 1298:265-72. DOI: 10.17660/ActaHortic.2020.1298.36 DOI: https://doi.org/10.17660/ActaHortic.2020.1298.36
Rittirat S, Thammasiri K, Klaocheed S. 2021. In vitro rapid multiplication of a highly valuable ornamental aquatic plant Anubias heterophylla. Trends Sci 18(19):3. DOI: 10.48048/tis.2021.3 DOI: https://doi.org/10.48048/tis.2021.3
Romano A, Noronha C, Martins-Loução MA. 1992. Influence of growth regulators on shoot proliferation in Quercus suber L. Annals of Botany. 70: 531–536. DOI: 10.1093/oxfordjournals.aob.a088513 DOI: https://doi.org/10.1093/oxfordjournals.aob.a088513
Saiprasad GVS, Raghuveer P, Khetrapal S, Chandra R. 2002. Effect of various growth regulators on the production of protocorm-like bodies in three orchid genera. Indian J Plant Physiol 7:35-9.
Sarma B, Tanti B. 2017. In vitro regeneration of plantlets from nodal explants of Aristolochia saccata and Aristolochia cathcartii. Eur J Biol Res 7:191-201. DOI: 10.5281/zenodo.825746
Sheeja GE, Joseph A, Korath A. 2015. In vitro propagation of an ornamental aquatic plant, Anubias barteri var. nana Petite. Int J Current Sci 18:1-12.
Sheelavanthmath SS, Murthy HN, Hema BP, Hahn EJ, Paek KY. 2005. High frequency of protocorm-like bodies (PLBs) induction and plant regeneration from protocorm and leaf sections of Aerides crispum. Sci Hortic 106(3):395-401. DOI: 10.1016/j.scienta.2005.04.012 DOI: https://doi.org/10.1016/j.scienta.2005.04.012
Sholichah L, Yamin M, Ginanjar R, Meilisza N. 2020. Anubias (Anubias sp.) propagation through hydroponic culture technique. In Journal of Physics: Conference Series 1422(1):012024. IOP Publishing.DOI: 10.1088/1742-6596/1422/1/012024 DOI: https://doi.org/10.1088/1742-6596/1422/1/012024
Surendra B, Aasim M, Fatih ÖS, Sebahattin Ö. 2019. Effect of gibberellic acid on in vitro flowering from stem node explant of Anubias barteri var. nana. Res J Biotechnol 14:.
Yu YX, Liu LL, Liu JX, Wang JW. 2009. Plant regeneration by callus-mediated protocorm-like body induction of Anthurium andraeanum Hort Agr Sci China 8(5):572-7. DOI: 10.1016/S1671-2927(08)60248-5 DOI: https://doi.org/10.1016/S1671-2927(08)60248-5
Copyright (c) 2024 Phong V. Nguyen, Giang K. T. Le, Tinh V. Nguyen, Ngan K. T Tran, Vy T. H. Dang

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree with the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work 1 year after publication simultaneously licensed under a Creative Commons attribution-noncommerical-noderivates 4.0 International License that allows others to share, copy and redistribute the work in any medium or format, but only where the use is for non-commercial purposes and an acknowledgement of the work's authorship and initial publication in this journal is mentioned.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).