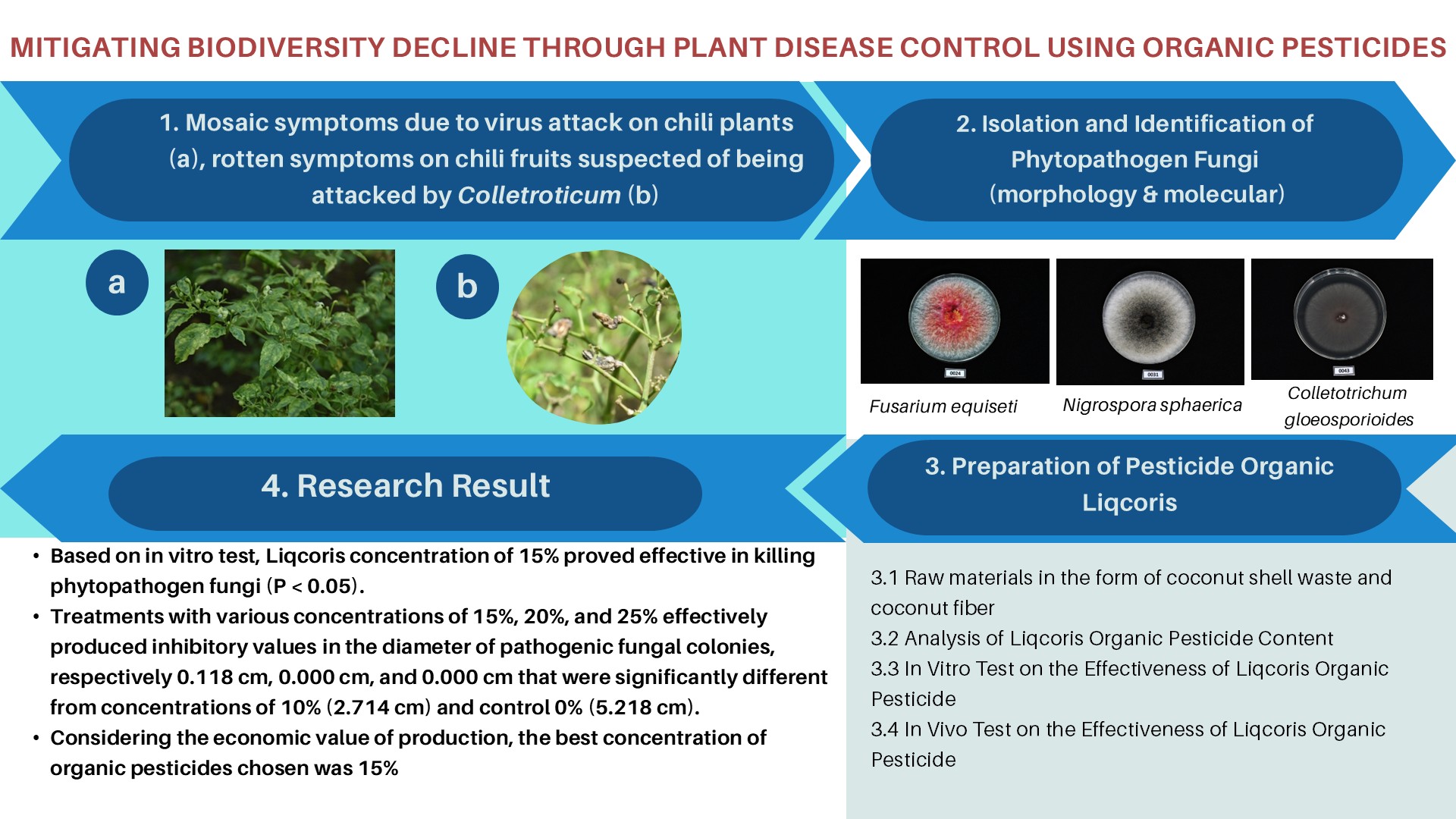
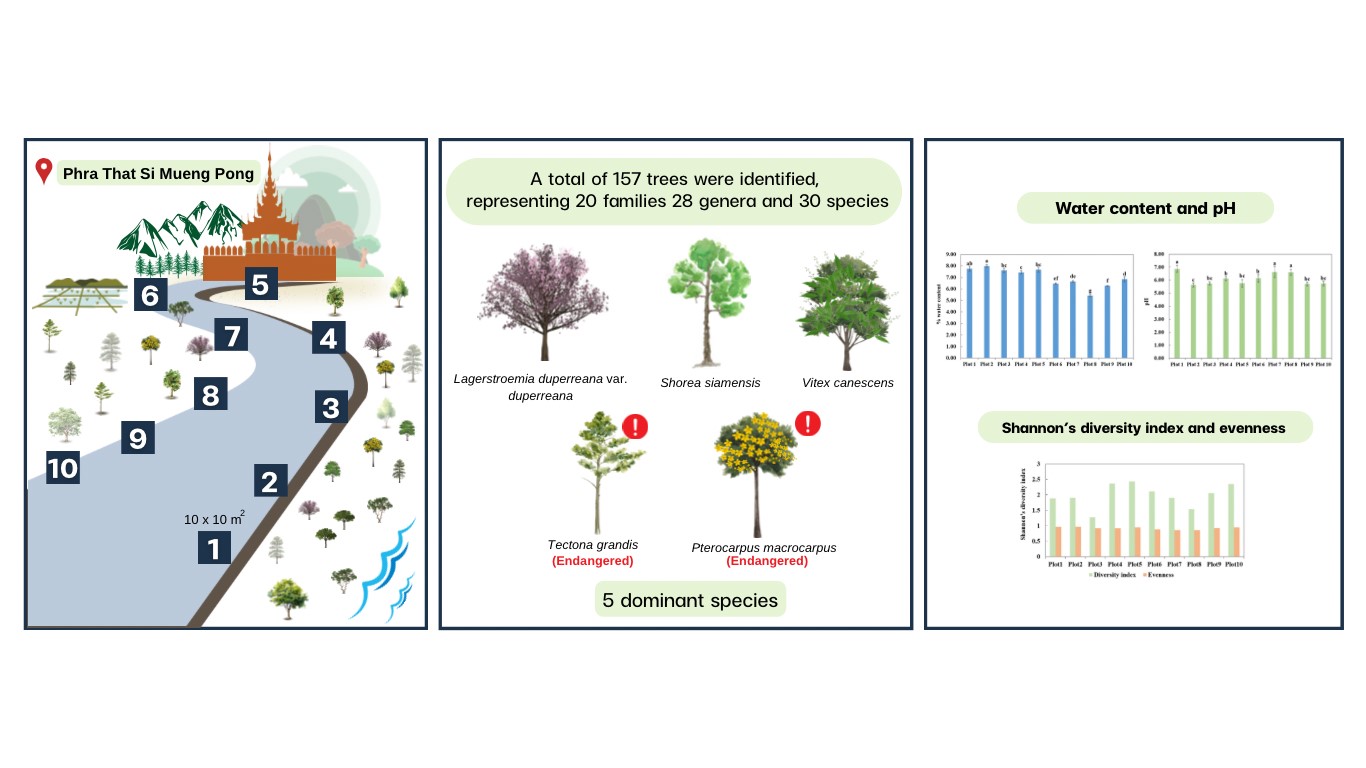
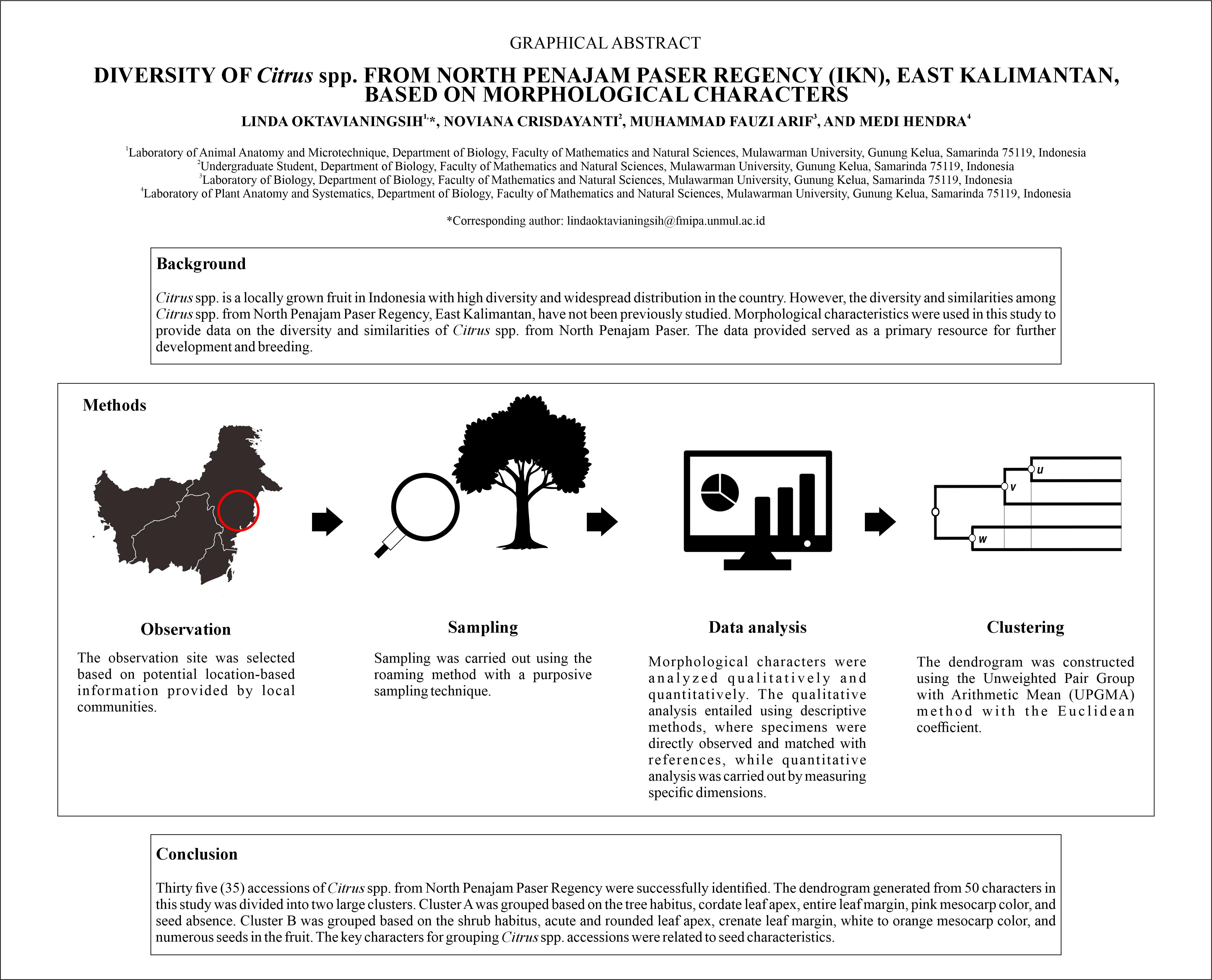
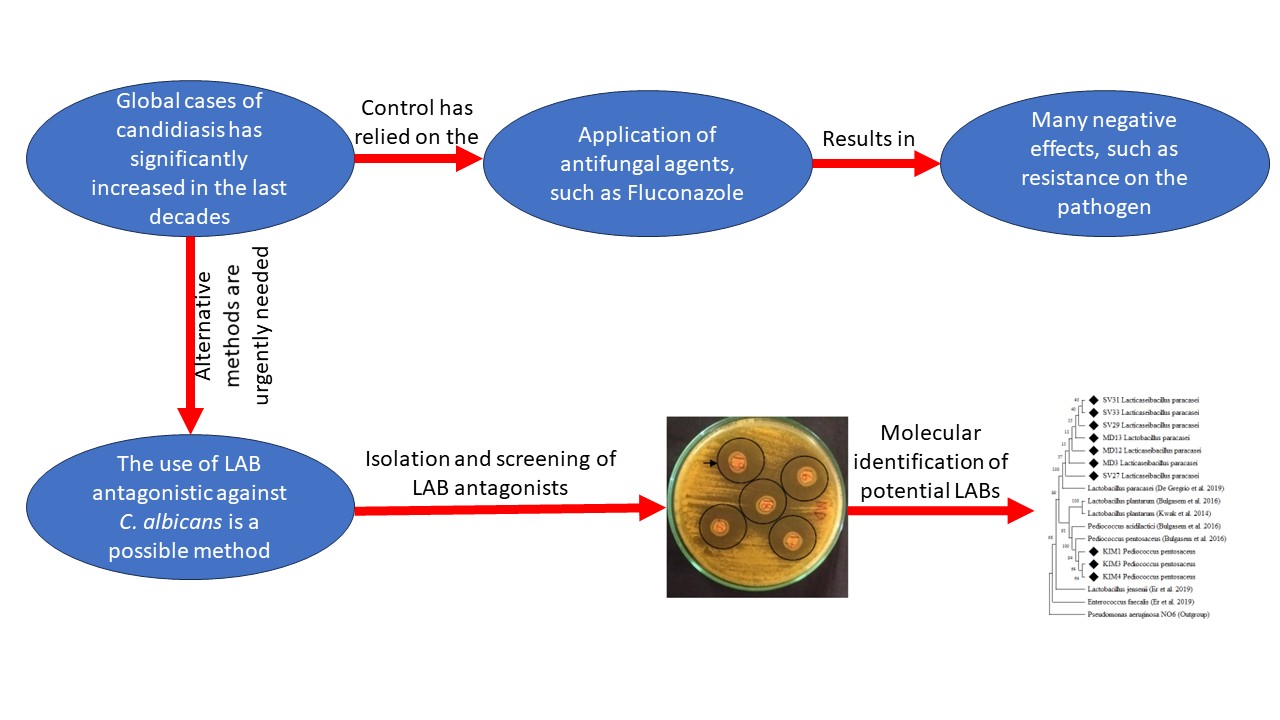
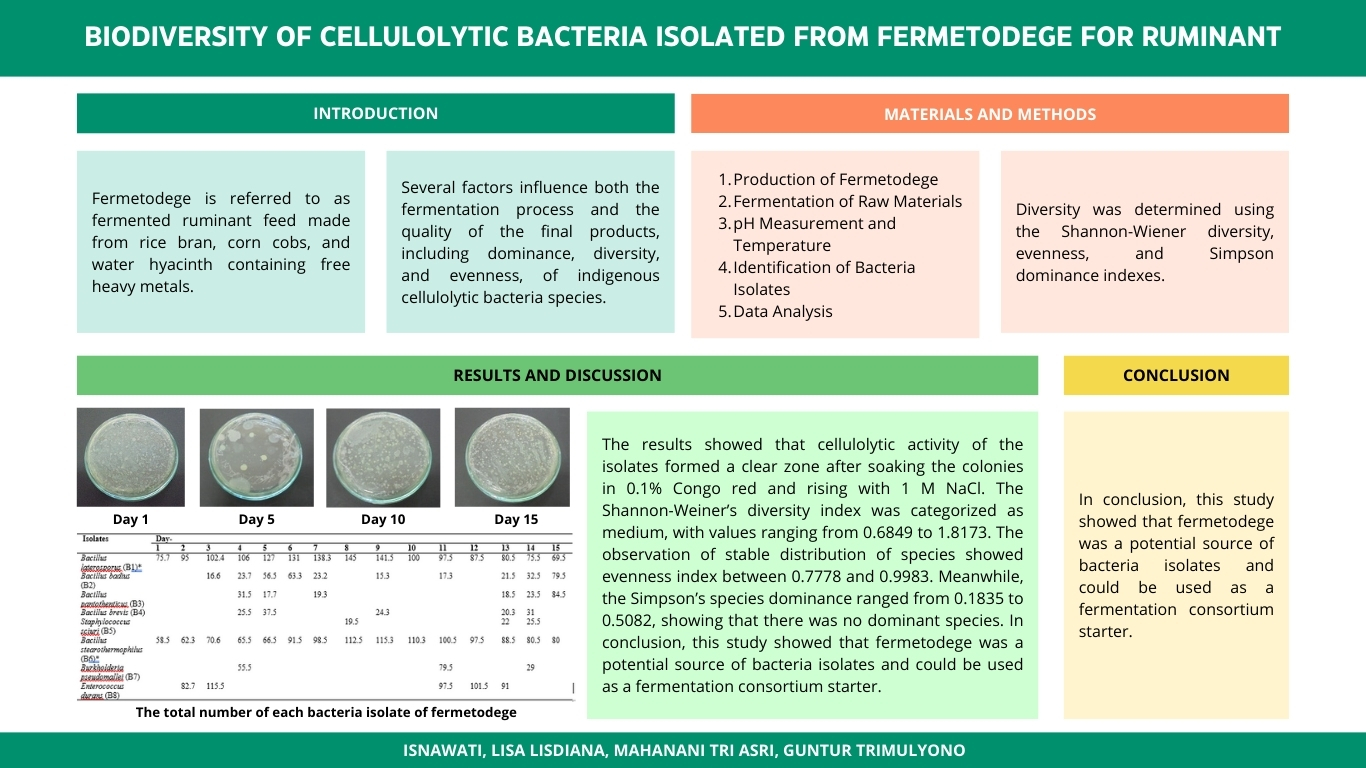
BIODIVERSITY OF CELLULOLYTIC BACTERIA ISOLATED FROM FERMETODEGE FOR RUMINANTS
Downloads
ARTICLE HIGLIGHTS
- Fermetodege as a source of cellulolytic bacteria that degrade cellulosic materials.
- Enhances ruminant feed by improving palatability and digestibility.
- Utilizes water hyacinth, reducing invasive plant spread and supporting ecosystems.
- Bioprospecting reveals new bacteria for effective fermentation starter development.
ABSTRACT
Fermetodege is a name of fermented ruminant feed produced from free heavy metals water hyacinth, rice bran, and corn cobs. Its quality, as well as the fermentation process, are affected by numerous factors, such as the diversity, evenness, and dominance of indigenous cellulolytic bacterial species. A proper understanding of these influential factors is needed to improve the quality of the fermented feed. Therefore, this study aims to evaluate the diversity, evenness, and dominance of cellulolytic bacteria isolated from fermetodege. The cellulolytic activity of the isolates was tested by observing their growth on the carboxymethylcellulose (CMC) media. Isolates with cellulolytic activity formed a clear zone after the colonies were soaked with 0.1% Congo red and rinsed with 1 M NaCl. The Shannon-Weiner's diversity and evenness indexes, as well as Simpson's species dominance, were then calculated. The result showed that the Shannon-Weiner’s diversity index ranged from 0.6849 to 1.8173, and it was categorized as medium. The evenness index was between 0.7778 and 0.9983, which indicates a stable distribution of species. Meanwhile, the Simpson’s species dominance ranged from 0.1835 to 0.5082, which implies that none of the species was dominant. These results show that fermetodege is potentially a source of bacterial isolates and can be used as a fermentation consortium starter.
Downloads
Adanikin BA, Ogunwande GA, Adesanwo OO. 2017. Evaluation and kinetics of biogas yield from morning glory (Ipomoea aquatica) co-digested with water hyacinth (Eichhornia crassipes). Ecol Eng 98:98-104. DOI: 10.1016/j.ecoleng.2016.10.067 DOI: https://doi.org/10.1016/j.ecoleng.2016.10.067
Aldrich GC, Koppel K. 2015. Pet food palatability evaluation: A review of standard assay techniques and interpretation of results with a primary focus on limitations. Animals (Basel) 5(1):43-55. DOI: 10.3390/ani5010043 DOI: https://doi.org/10.3390/ani5010043
Atta M, Mahgoup O, Al-Marri SJ, Al-Hajri F. 2018. Evaluation of digestibility and feed intake of a date palm by-products diets fed to Awassi rams. Research Opinions in Animal and Veterinary Sciences (ROAVS) 8(2):29-36.
Akita H, Kimura Z, Zulkhairi M, Yusoff M, Nakashima N, Hoshino T. 2016. Isolation and characterization of Burkholderia sp. strain CCA53 exhibiting ligninolytic potential. Springer Plus 5: 596-600. DOI: 10.1186/s40064-016-2237-y DOI: https://doi.org/10.1186/s40064-016-2237-y
Belewu, MA, Babalola FT. 2009. Nutrient enrichment of waste agricultural residues after solid state fermentation using Rhizopus oligosporus. Biotechnol Biofuels 13:695-9. Available from: http://www.biosciences.elewa.org/JABS
Bhatia A, Rajpal A, Madan S, Kazmi AA. 2015. Techniques to analyze diversity during composting: A mini review. Indian Journal of Biotechnology 14:19-25. Available from: https://www.semanticscholar.org/paper/Techniques-to-analyze-microbial-diversity-during-BhatiaRajpal/41e2fadaba67cd18b87e5869483345f2fe29f 2c6
Boboescu IZ, Ilie M, Gherman VD, Mirel I, Pap B, Negra A, …, Biro T, Maroti G. 2014. Revealing the factors influencing a fermentative biohydrogen production process using industrial wastewater as fermentation substrate. Biotechnol Biofuels 7:139-54. DOI: 10.1186/s13068-014-0139-1 DOI: https://doi.org/10.1186/s13068-014-0139-1
Croos AMB, Rajendran S, Ranganathan K. 2019. Isolation of a cellulase producing Bacillus cereus from cow dung and determination of the kinetic properties of the crude enzyme. Journal of the National Science Foundation of Sri Lanka (JNSF) 47(2):261-7. DOI: 10.4038/jnsfsr.v47i2.9168 DOI: https://doi.org/10.4038/jnsfsr.v47i2.9168
Dantur KI, Enrique R, Welin B, Castagnaro AP. 2015. Isolation of cellulolytic bacteria from the intestine of Diatraea saccharalis larvae and evaluation of their capacity to degrade sugarcane biomass. AMB Express 5 :1-11. DOI: 10.1186/s13568-015-0101-z DOI: https://doi.org/10.1186/s13568-015-0101-z
Dezam APG, Vasconcellos VM, Lacava PT, Farinas CS. 2017. Microbial production of organic acids by endophytic fungi. Biocatalysis and Agricultural Biotechnology 11:282-7. DOI: 10.1016/j.bcab.2017.08.001 DOI: https://doi.org/10.1016/j.bcab.2017.08.001
Farida Y, Sasongko H, Sugiyarto. 2018. Utilization of local as fermented feed and supplements for livestock in Sendang Village, Wonogiri District. Agrokreatif 4:61-7. DOI: DOI: 10.29244/agrokreatif.4.1.61-67 DOI: https://doi.org/10.29244/agrokreatif.4.1.61-67
Fitrihidajati H, Ratnasari E, Isnawati, Soeparno G. 2015. Quality of fermentation result of ruminant feed production made of water hyacinth (Eichornia crassipes). Journal of Biosantifika 7:62-7. DOI: 10.15294/biosaintifika.v7i1.3540
Fitrihidajati H, Isnawati ER. 2017. Effectiveness of ruminant feed formula from the fermented water hyacinth (Eichhornia crassipes) to produce the high level protein of goat meat. Adv Sci Lett 23:11972-5. DOI:10.1166/asl.2017.10555 DOI: https://doi.org/10.1166/asl.2017.10555
Gaur R, Tiwari S. 2015. Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol 15:19. DOI: 10.1186/s12896-015-0129-9 DOI: https://doi.org/10.1186/s12896-015-0129-9
Hanif MA, Siddik MAB, Chaklader MR, Nahar A, Mahmud S. 2015. Fish diversity in the southern coastal waters of Bangladesh: Present status, threats and conservation perspectives. Croatian Journal of Fisheries: Ribarstvo 73:148-61. DOI: 10.14798/73.4.848 DOI: https://doi.org/10.14798/73.4.848
Hansen GH, Lübeck M, Frisvad JC, Lübeck PS, Andersen B. 2015. Production of cellulolytic enzymes from Ascomycetes: Comparison of solid state and submerged fermentation. Process Biochemistry 50:1327-41. DOI: 10.1016/j.procbio.2015.05.017 DOI: https://doi.org/10.1016/j.procbio.2015.05.017
Holt JG. 1994. Bergey's Manual of Determinative Bacteriology. Baltimore (US): Williams & Wilkins.
Hossain ME, Sikder H, Kabir MH, Sarma SM. 2015. Nutritive value of water hyacinth (Eichhornia crassipes). J Anim Feed Res 5:40-4. Available from: http://www.science-line.com/index/; http://www.ojafr.ir
Ishii K, Fukui M, Takii S. 2000. Microbial Succession during a composting process as evaluated by denaturing gradient gel electrophoresis analysis. J Appl Microbiol 89:768-77. DOI: 10.1046/j.1365-2672.2000.01177.x DOI: https://doi.org/10.1046/j.1365-2672.2000.01177.x
Isnawati. 2019. Cellulolytic activity of indigenous fungi in fermentation: ruminant fermented feed made of water hyacinth (Eichhornia crassipes) and corn cobs (Zea mays). Jurnal of Biological Research and Its Applications 1:26-31.
Isnawati. 2020. Feed sheep (Ovis aries) fermentation technology of water hyacinth (Eichhornia crassipes) and corn (Zea mays) cob with the indigenous microorganisms consortium [Dissertation]. Surabaya (ID): Airlangga University. (in Indonesian).
Istiqomah L, Febrisiantosa A, Sofyan A, Damayanti E. 2010. Implementation of fermented rice bran as a flavor enhancer additive and its effect on feed utilization and beef cattle performance. Article presented article in the 5th International Seminar on Tropical Animal Production Community Empowerment and Tropical Animal Industry. Yogyakarta, Indonesia, 19-22 October 2010.
Lardy G, Anderson V, Dahlen C, Carlson Z.2022. Alternative feeds for ruminants.. North Dakota (US): NDNU extension.
Leite P, Sousa D, Fernandes H, Ferreira M, Costa AR, Filipe D, …, Salgado JM. 2020. Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Current Opinion in Green and Sustainable Chemistry 100407. DOI: 10.1016/j.cogsc.2020.100407 DOI: https://doi.org/10.1016/j.cogsc.2020.100407
Liang YL, Zhang Z, Wu M, Wu Y, Feng JX. 2014. Isolation, screening, and identification of cellulolytic bacteria from natural reserves in the subtropical region of China and optimization of cellulase production by Paenibacillus terrae ME27-1. BioMed Research International 2014: 12 pgs. DOI: 10.1155/2014/512497 DOI: https://doi.org/10.1155/2014/512497
Liu YC, Zhou X, Dong Q, Shi H. 2015. Study of the succession of microbial communities for sulfur cycle response to ecological factors change in sediment of sewage system. Environ Sci Pollut Res 22:9250-9. DOI: 10.1007/s11356-014-3934-0 DOI: https://doi.org/10.1007/s11356-014-3934-0
Liu L, Wen W, Zhang R, Wei Z, Deng Y, Xiao J, Zhang M. 2017. Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem 214: 1-8. DOI: 10.1016/j.foodchem.2016.07.038 DOI: https://doi.org/10.1016/j.foodchem.2016.07.038
Lynd LR, Heber van Zyl W, Pretorius IS, Weimer PJ. 2002. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol Mol Biol Rev 66 :506-77. DOI: 10.1128/MMBR.66.4.739.2002. DOI: https://doi.org/10.1128/MMBR.66.3.506-577.2002
Oke MA, Annuar MSM, Simarani K. 2016. Enhanced endoglucanase production by Bacillus aerius on mixed lignocellulosic substrates. Bioresources 11:5854-69. Available from: http://ncsu.edu/bioresources DOI: https://doi.org/10.15376/biores.11.3.5854-5869
Ortolani IR, Amanzougarene Z, Fondevila M. 2020. In vitro estimation of the effect of grinding on rumen fermentation of fibrous feeds. Animals (Basel) 23:10(4):732. DOI: 10.3390/ani10040732 DOI: https://doi.org/10.3390/ani10040732
Pakpahan S, Restiani R. 2019. The efect of fermented complete feed based on local feed resources on weight gain of female peranakan etawah goats in Samigaluh Subdistrict. AIP Conference Proceedings 2199, 050009 (2019). DOI: 10.1063/1.5141307 DOI: https://doi.org/10.1063/1.5141307
Paynor KA, David ES, Valentino MJG. 2016. Endophytic fungi associated with bamboo as possible sources of single cell protein using corn cob as a substrate. Mycosphere 7:139-47. DOI: 10.5943/mycosphere/7/2/5 DOI: https://doi.org/10.5943/mycosphere/7/2/5
Ramos CL, de Almeida EG, Freire AL, Schwan RF. 2011. Diversity of bacteria and yeast in the naturally fermented cotton seed and rice beverage produced. Food Microbiol 28:1380-6. DOI: 10.1016/j.fm.2011.06.012 DOI: https://doi.org/10.1016/j.fm.2011.06.012
Rezania S, Din MFM, Taib SM, Mohamad SE, Dahalan FA, Kamyab H, …, Ebrahimi SS. 2018. Ethanol production from water hyacinth (Eichhornia crassipes) using various types of enhancers based on the consumable sugars. Waste and Biomass Valorization 9:939-46. DOI: 10.1007/S12649-017-9883-3 DOI: https://doi.org/10.1007/s12649-017-9883-3
Sadh PK, Duhan S, Duhan JS. 2018. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour Bioprocess 5:1-15. DOI: 10.1186/s40643-017-0187-z DOI: https://doi.org/10.1186/s40643-017-0187-z
Shariff A, Mohamad Aziz NS, Ismail NI, Abdullah N. 2016. Corn cob as a potential feedstock for slow pyrolysis of biomass. J Phys Sci 27:123-37. DOI: 10.21315/jps2016.27.2.9 DOI: https://doi.org/10.21315/jps2016.27.2.9
Silva-Mendoza J, Gómez-Treviño A, Lopez-Chuken U, Blanco-Gámez EA, Chávez-Guerrero L, Cantú-Cárdenas, ME. 2020. Agave leaves as a substrate for the production of cellulases by Penicillium sp. and the obtainment of reducing sugars. J Chem 2020. DOI: 10.1155/2020/6092165 DOI: https://doi.org/10.1155/2020/6092165
Sivaramakrishnan R, Ramprakash B, Ramadoss G, Suresh S, Pugazhendhi A, Incharoensakdi A. 2021. High potential of Rhizopus treated rice bran waste for the nutrient-free anaerobic fermentative biohydrogen production. Bioresour Technol 319:124-93. DOI: 10.1016/j.biortech.2020.124193 DOI: https://doi.org/10.1016/j.biortech.2020.124193
Sundarajan S, Kannan C, Chittibabu S. 2011. Alkaline protease from Bacillus cereus VITSN04: potential application as a dehairing agent. J Biosci Bioeng 111 (2):128-33. DOI: 10.1016/j.biortech.2020.124193 DOI: https://doi.org/10.1016/j.jbiosc.2010.09.009
Tabssum F, Irfan M, Shakir HA, Qazi JI. 2018. RSM based optimization of nutritional conditions for cellulase mediated saccharification by Bacillus cereus. J Bio Eng 12:1-10. DOI: 10.1186/s13036-018-0097-4 DOI: https://doi.org/10.1186/s13036-018-0097-4
Taherzadeh MJ, Karimi K. 2008. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int J Mol Sci. 9:1621-51. DOI: 10.3390/ijms9091621 DOI: https://doi.org/10.3390/ijms9091621
Tang A, Haruna AO, Majid MA. 2018. Potential PGPR properties of cellulolytic, nitrogen-fixing, and phosphate-solubilizing bacteria of a rehabilitated Tropical Forest Soil. bioRxiv 20. DOI: 10.1101/351916 DOI: https://doi.org/10.1101/351916
Varma VS, Ramu K, Kalamdhad AS. 2015. Carbon decomposition by inoculating Phanerochaete chrysosporium during drum composting of agricultural waste. Environ Sci Pollut Res 22:7851-8. DOI: 10.1007/s11356-014-3989-y DOI: https://doi.org/10.1007/s11356-014-3989-y
Vial L, Groleau M, Dekimpe V, Deziel E. 2007. Burkholderia diversity and versatility: an inventory of the extracellular products. J Microbiol Biotechnol 17(9):1407-29.
Yu H, Zeng G, Huang H, Xi X, Wang R, Huang D, …, Li J. 2007 Microbial community succession and lignocellulose degradation during agricultural waste composting. Biodegradation 18:793-802. DOI: 10.1007/s10532-007-9108-8 DOI: https://doi.org/10.1007/s10532-007-9108-8
Yu M, Zhang J, Xu Y, Xiao H, An W, Xi H, …, Shen A. 2015. Fungal community dynamics and driving factors during agricultural waste composting. Environ Sci Pollut Res 22:19879-86.DOI: 10.1007/s11356-015-5172-5 DOI: https://doi.org/10.1007/s11356-015-5172-5
Copyright (c) 2024 Isnawati, Lisa Lisdiana, Mahanani Tri Asri, Guntur Trimulyono

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree with the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work 1 year after publication simultaneously licensed under a Creative Commons attribution-noncommerical-noderivates 4.0 International License that allows others to share, copy and redistribute the work in any medium or format, but only where the use is for non-commercial purposes and an acknowledgement of the work's authorship and initial publication in this journal is mentioned.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).