6 – BENZYLAMINOPURINE INDUCES HIGH-FREQUENCY MULTIPLICATION IN VULNERABLE Curcuma pseudomontana J. Graham.: A POTENTIAL EX VIVO CONSERVATION TOOL
Downloads
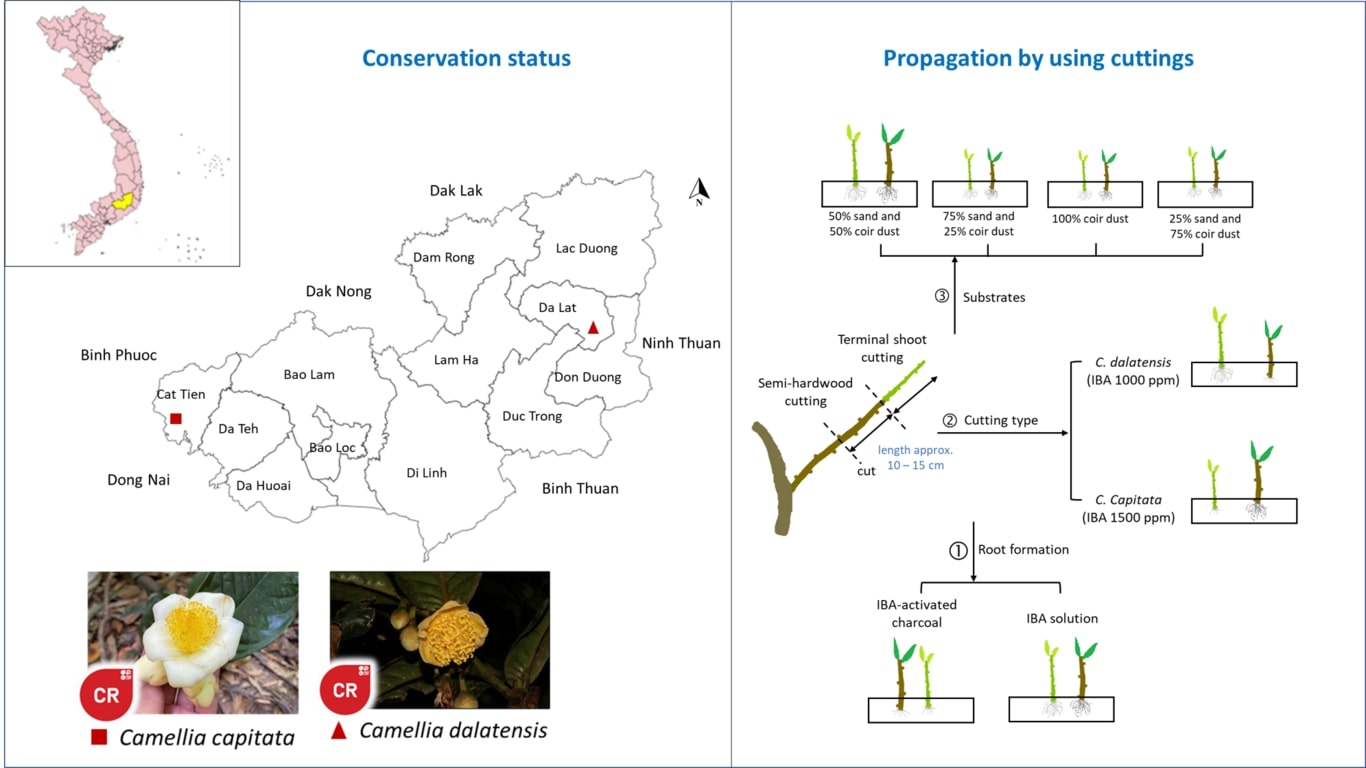
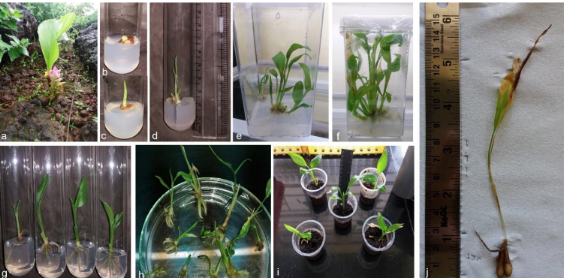
A rapid high-frequency multiplication protocol is designed for Curcuma pseudomontana J. Graham, belonging to the family Zingiberaceae, an endemic species to the Western Ghats of India. The IUCN Red List of Threatened Taxa mentions this species as vulnerable due to multiple underlying causes. The Plant is extensively used in traditional and tribal medicine. The species has suffered habitat loss due to uncontrolled use for tribal medicine leading to a 30% loss in the last decade. This study is planned with a specific objective to conserve the species, and this is the first-ever report of micropropagation of Curcuma pseudomontana J. Graham using in vitro multiplication. An efficient rapid protocol for Micropropagation is developed using rhizome bud explants. The explants are transferred from MS basal medium onto the MS medium fortified with BAP, KN, TDZ at a varying concentration range. The maximum shoot induction is observed in MS medium enriched with BAP 2mg L-1resulting in 9.66 ±2.08 number of shoots per explant with a shoot length of 6.40 ±0.36cm. The root induction response is studied by aseptically transferring the shoots onto MS medium fortified with NAA, IBA, and IAA at varying concentration. Maximum root length and root number is recorded in MS supplemented with 1- Naphthalene Acetic Acid (NAA) at 0.5 mg L-1. However, a 100 % root induction frequency is observed in all the samples under study. The rooted plantlets are removed from the culture flasks and transferred into hardening media containing 1:1:1 ratio of Sand: Soil: Cocopeat. The hardened plants are healthy and disease-free and showed a 92% survival after acclimatization.
Downloads
Barbara MR, Viswambharan S, Michaek K, Eric B & Valerie CP. 2011. Biodiversity conservation and conservation biotechnology tools. In vitro Cellular & Developmental Biology -Plant.47: 1-4. DOI: https://doi.org/10.1007/s11627-010-9337-0
Bawa KS, Das A, Krishnaswamy J, Karanth KU, Kumar NS and Rao M. 2007. Ecosystem Profile: Western Ghats and Sri Lanka Biodiversity Hotspot - Western Ghats Region. Critical Ecosystems Partnership Fund, Virginia.
Bejoy M, Dan M, Anish NP. 2006. Factors affecting the in vitro multiplication of the endemic zingiber Curcuma haritha Mangaly and Sabu. Asian J. Plant Sci.5(5): 847-53. DOI: https://doi.org/10.3923/ajps.2006.847.853
Bejoy M, Dan M, Anish NP, Nair AR, Radhika BJ, Manesh K.2012. Micropropagation of an Indian Ginger (Curcuma vamana Sabu and Mangaly): a wild relative of turmeric. Biotechnology. 11(6): 333-8. DOI: https://doi.org/10.3923/biotech.2012.333.338
Bharalee R, Das A, Kalita MC. In vitro clonal propagation of Curcuma caesia Roxb and Curcuma zedoaria Rosc from rhizome bud explants.2005. Journal of Plant Biochemistry and Biotechnology. 14: 61-3. DOI: https://doi.org/10.1007/BF03263228
Bisht V.S., Kandwal S, Kanan D, Som D. 2014. Anticancerous and antiproliferative/cytotoxic activity of Curcuma pseudomontana (hill turmeric) collected from the sub-Himalayan region of Uttrakhand, India. Asian Journal of Plant Science and Research.4(6): 25-31.
Hiremath GB, and Kaliwal BB. 2013. Antitubercular activity of the rhizome of Curcuma pseudomontana J. Graham. International Journal of Pharmaceuticals and Health care Research, Int. J. Pharm. & H. Care Res.01 (04): 178-183.
Hiremath GB, Kaliwal BB. 2014. Pharmacognostic evaluation of rhizome of Curcuma pseudomontana j. Graham. International Journal of Pharma and Bio Sciences, Int J pharm bio sci. 5(2): 242-250.
Jagtap SD, Deokule SS, Bhosle SV. 2006. Some unique ethnomedicinal uses of plants used by the Korku tribe of Amravati district of Maharashtra, India. Journal of Ethnopharmacology. 107: 463-469. DOI: https://doi.org/10.1016/j.jep.2006.04.002
Koarapatchaikol K, Kanmarangkol S, Kaewraksa J. 2017. In vitro Plant Regeneration via Callus Culture in Turmeric (Curcuma longa L.) Burapha Science Journal.22(1).
Kou Y, Ma G, Silva JA. 2013. Callus induction and shoot organogenesis from anther cultures of Curcuma attenuata Wall. Plant Cell and Organ Culture. 112:1-7. DOI: https://doi.org/10.1007/s11240-012-0205-y
Loc NH, Duc DT, Kwon TH, & Yang MS, 2005. Micropropagation of zedoary (Curcuma zedoaria Roscoe)–a valuable medicinal plant. Plant cell, tissue and organ culture.81: 119-122. DOI: https://doi.org/10.1007/s11240-004-3308-2
Mohanty S, Panda MK, Subudhi E, Nayak S. 2008. Plant regeneration from callus culture of Curcuma aromatica and in vitro detection of somaclonal variation through cytophotometric analysis. Biologia Plantarum.52(4): 783-786. DOI: https://doi.org/10.1007/s10535-008-0153-x
Molur S, Walker S. 1997. Conservation Assessment and Management Plan Workshop.
Murashige T, & Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15(3): 473-497. DOI: https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nayak S. 2000. In vitro multiplication and microrhizome induction in Curcuma aromatica Salisb. Plant Growth Regulation. 32(1): 41-7. DOI: https://doi.org/10.1023/A:1006307316393
Nayak S, Naik PK. 2006. Factors effecting in vitro microrhizome formation and growth in Curcuma longa L. and improved field performance of micropropagated plants. Science Asia.32(1): 31-7. DOI: https://doi.org/10.2306/scienceasia1513-1874.2006.32.031
Prasad M.N. V, Padmalatha, Jayaram K., Raju N.L. 2006. Healing plants of Andhra Pradesh-indigenous knowledge, trade, threats, and conservation strategies. Proc.A.P.Academi of Sciences. 10(2): 109-120.
Prakash S., Elangomathavan R., Seshadri S., Kathairavan K., Ignacimuthu. 2004. Efficient Regeneration of Curcuma amada Roxb. Plantlets from Rhizome and Leaf Sheath Explants. Plant Cell, Tissue and Organ Culture.78(2): 159-165. DOI: https://doi.org/10.1023/B:TICU.0000022553.83259.29
Raihana R, Faridah QZ, Julia AA, Abdelmageed AH, Kadir MA.2011. In vitro culture of Curcuma mangga from rhizome bud. Journal of Medicinal Plant Research. 5(28): 6418-22. DOI: https://doi.org/10.5897/JMPR11.673
Ravikumar K, Ved DK, assisted by Vijaya Sankar. 2000. 100 Red Listed Medicinal Plants of conservation concern in Southern India-Illustrated field guide.
Ravindran PN, Babu KN, Sivaraman K, editors. 2007. Turmeric: the genus Curcuma.CRC press. DOI: https://doi.org/10.1201/9781420006322
Romand-Monnier, F. & Contu, S. 2013. Curcuma pseudomontana. The IUCN Red List of Threatened Species 2013: e.T22486190A44506743. DOI: 10.2305/ IUCN.UK.2013-1.RLTS. T22486190A44506743.en.
Sabu M. 2006 Zingiberaceae and Costaceae of South India, Indian Association of Angiosperm taxonomy.
Salvi ND, George L, Eapen S. 2000. Direct regeneration of shoots from immature inflorescence cultures of turmeric. Plant Cell, Tissue and Organ Culture.62: 235-238. DOI: https://doi.org/10.1023/A:1006459822879
Salvi ND, George L, Eapen S. 2001. Plant regeneration from leaf base callus of turmeric and random amplified polymorphic DNA analysis of regenerated plants. 2001.Plant Cell,Tissue and Organ Culture.66: 13-119. DOI: https://doi.org/10.1023/A:1010638209377
Santapau H. 1945. Curcuma pseudomontana Grah. Journal of the Bombay Natural History Society 45: 618-623.
Sasikumar B. 2005. Genetic resources of Curcuma: diversity, characterization and utilization. Plant Genetic Resources. 3(2): 230-51. DOI: https://doi.org/10.1079/PGR200574
Shanthala AA, Dilkalal A, Umesh TG. 2021. An Efficient Invitro Approach for Direct and Callus Mediated Regeneration of Curcuma karnatakensis–An Endemic Plant of Karnataka. Journal of Herbs, Spices & Medicinal Plants.27(3): 229-41. DOI: https://doi.org/10.1080/10496475.2020.1806166
Shirgurkar MV, John CK, Nadgauda RS. 2001. Factors affecting in vitro microrhizome production in turmeric. Plant Cell, Tissue and Organ Culture. 64: 5-11. DOI: https://doi.org/10.1023/A:1010645624618
Sundram., Suffian M. Annuar M., Khalid N. 2012. Optimization of culture condition for callus induction from shoot buds for establishment of rapid growing cell suspension cultures of Mango ginger (Curcuma mangga) Tamil C.M. AJCS. 6(7): 1139-1146.
Theanphing O, Songsak T, Kerdmanee C. 2010. Effect of plant growth regulators on micropropagation of Curcuma aeruginosa Roxb.Thai journal of botany. 2: 135-142.
Toppoonyanont N, Chongsang S, Chujan S, Somsueb S, Nuamjaroen P. 2004. Micropropagation Scheme of Curcuma alismatifolia Gagnep. InIX International Symposium on Flower Bulbs. 673(98): 705-712. DOI: https://doi.org/10.17660/ActaHortic.2005.673.98
Ugochukwu S., Bob E. 2013. Shoot Proliferation of In vitro Turmeric (Curcuma longa L.) Affected by Different Concentrations of Benzylaminopurine. World Journal of Agricultural. 9(3): 227-230.
Yasuda K, Tsuda T, Shimizu H, Sugaya A. 1988. Multiplication of Curcuma species by tissue culture. Planta Med. 54(1): 75-79. DOI: https://doi.org/10.1055/s-2006-962344
Zhang S, Liu N, Sheng A, Ma G, Wu G. 2011. In vitro plant regeneration from organogenic callus of Curcuma kwangsiensis Lindl. (Zingiberaceae).Plant Growth Regulation. 64(2): 141-145. DOI: https://doi.org/10.1007/s10725-010-9548-8
Copyright (c) 2024 Gayatri Vaze, Pramod Hurkadale, Harsha Hegde

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree with the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work 1 year after publication simultaneously licensed under a Creative Commons attribution-noncommerical-noderivates 4.0 International License that allows others to share, copy and redistribute the work in any medium or format, but only where the use is for non-commercial purposes and an acknowledgement of the work's authorship and initial publication in this journal is mentioned.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).