TICK-BORNE PATHOGENS DETECTION FROM TICKS INFESTING Malayopython reticulatus (REPTILIA: PYTHONIDAE) SNAKES IN INDONESIA
Article Highlights:
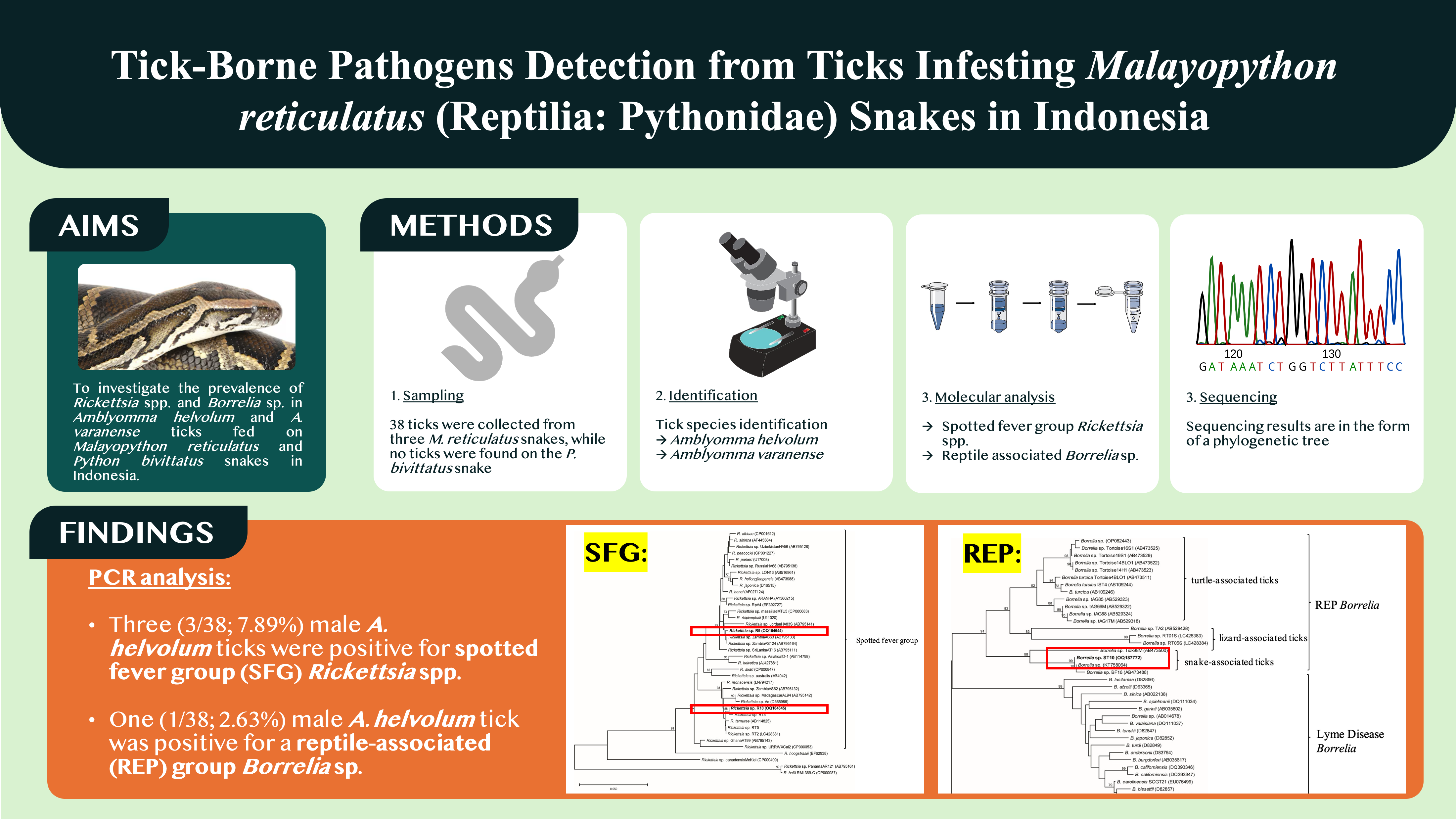
- A total of 38 ticks were collected from M. reticulatus, comprising 13 A. helvolum and 25 A. varanense.
- Spotted fever group Rickettsia spp. (7.89%) and reptile-associated Borrelia sp. (2.63%) were detected in male Amblyomma helvolum ticks collected from Malayopython reticulatus snakes in Indonesia.
- Snake-associated ticks may harbor emerging pathogens, underscoring the importance of tick surveillance in reptiles for early disease detection and zoonotic prevention.
Abstract:
Ticks are important arthropod vectors of numerous diseases in humans and animals. Furthermore, ticks are also established vectors and reservoirs of pathogens important to wildlife and human health. Rickettsia and Borrelia are two genera of bacteria that may be transmitted by ticks, and some pathogenic species are zoonosis. This research investigated the prevalence of Rickettsia spp. and Borrelia sp. in Amblyomma helvolum and Amblyomma varanense ticks fed on Malayopython reticulatus and Python bivittatus snakes in Indonesia. A total of 38 ticks were collected from three M. reticulatus snakes, while no ticks were found on the P. bivittatus snake. The 38 ticks consisted of 13 individuals A. helvolum and 25 individuals A. varanense. PCR analysis revealed that three (3/38; 7.89%) male A. helvolum ticks were positive for spotted fever group Rickettsia spp. and one (1/38; 2.63%) male A. helvolum tick was positive for a reptile-associated group Borrelia sp. Although the overall prevalence of tick-borne pathogens was low, this study underscores the importance of monitoring the prevalence and prevention of tick-borne diseases. Surveillance of ticks infesting reptiles can facilitate the early detection of disease transmission to both animals and humans. These findings also suggested that snake-associated ticks may harbor emerging tick-borne pathogens
Downloads
INTRODUCTION
Malayopython reticulatus (Reptilia: Pythonidae) is a non-venomous snake commonly found in Indonesia. This snake is also one of the wild animals susceptible to infestations by various ectoparasites, including ticks. Five tick species have been separately reported to infest M. reticulatus snakes, which are Amblyomma helvolum ((Anastos, 1950);(Anderson & Tzianabos, 1989);(Andoh et al., 2015)),
A. varanense(Anastos, 1950);(Andoh et al., 2015), A. cordiferum(Auffenberg, 1988), and Rhipicephalus sanguineus(Chao et al., 2013). Amblyomma latum was also reported to infest Python regius snakes in Ghana and Togo(Pandit et al., 2011);(Mariana et al., 2011);(Sumrandee et al., 2014);(Andoh et al., 2015).
Ticks known to be associated with pathogens include Ixodes spp., Rhipicephalus spp., Haemaphysalis spp., Amblyomma spp., and Dermacentor spp. ((Estrada-Peña & Jongejan, 1999);(Raoult et al., 2002);(Takano et al., 2014)). Several pathogens are associated with ticks, a few of which are Borrelia sp. and Rickettsia sp. The major groups of Borrelia impacting animal and human health are relapsing fever Borrelia, Lyme disease Borrelia, and reptile-associated (REP) Borrelia ((Bunikis & Barbour, 2005);(Takano et al., 2010);(Franke et al., 2013)). Borrelia spp. was previously detected in A. varanense infesting P. reticulatus in Thailand(Trinachartvanit et al., 2016). Meanwhile, Rickettsia sp. belonging to the spotted fever group (SFG) was also detected from A. transversale and A. trimaculatum ticks infesting P. regius and Boiga forsteni snakes, respectively(Andoh et al., 2015). This research aimed to determine the presence of Rickettsia spp. and Borrelia sp. in ticks that infest wild snakes in Indonesia. In addition, phylogenetic analyses of detected pathogens were also presented.
MATERIALS AND METHODS
Ticks were collected within the period of 2021- 2022 from three wild-caught M. reticulatus snakes in Bogor (6°35’42.1368’’ S and 106°48’59.8860’’ E) and Jakarta (20°58’50.736’’ N and 89°40’45.876’’ W) and one P. bivittatus snake found in Jakarta (20°58’50.736’’ N and 89°40’45.876’’ W). The snakes were handled in accordance with good animal welfare practices and released to their original habitats upon examination.
From the skin beneath their scales, ticks were collected using forceps and stored in 70% ethanol. Tick species, stage, and sex were identified based on morphologic features following taxonomic keys and molecular analysis(Anastos, 1950)(Kohls, 1957).
The collected ticks were washed individually and homogenized in 200 μL 10x PBS solution. Tick DNA was individually extracted using the DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Oligonucleotide primer pairs used in this study were 16SrDNA (mt-rrs 1 5’-CTGCTCAATGATTTTTTAAATTGCTGTGG-3’; mt-rrs 2 5’-CCGGTCTGAACTCAGATCAAGTA-3’) for tick identification, 17-kDa antigen (R1 5’-TCAATTCACAACTTGCCATT- 3’,R2 5’-TTTACAAAATTCTAAAAACC-3’) for Rickettsia detection, and flaB (PAD 5’-GATCARGCWCAAYATAACCAWATGCA-3’, PDU 5’-AGATTCA AGTCTGTTTTGGAAAGC-3’) for Borrelia detection ((Anderson & Tzianabos, 1989);(Takano et al., 2010)).
Amplifications were performed with the following conditions: 95 °C for 5 minutes, 94 °C for 45 seconds, 50-52 °C for 30 seconds, 72 °C for 45 seconds, and 72 °C for 10 minutes. PCR products were visualized using electrophoresis on 1.2% agarose gel stained with ethidium bromide in 1X TAE buffer. Electrophoresis was carried out at 100 V for 25 minutes. DNA from positive samples then amplified again as much as 50 μL for sequencing and the sample was sent to PT. Genetika Science Indonesia, Tangerang.
The sequences of positive samples were compared with sequences in the NCBI GenBank database by nucleotide BLAST. Phylogenetic analyses were performed using the MEGA7 software(www.megasoftware.net)(Tamura et al., 2007). The phylogenetic trees were constructed by the neighbor-joining method. Bootstrap analyses (1,000 replicates) were carried out according to the Kimura 2-parameter model. All sequences were deposited in GenBank (Accession numbers: Rickettsia sp. ST9 (OQ164644), Rickettsia sp. ST10 (OQ164645), Rickettsia sp. ST13 (OQ054254), and Borrelia sp. ST10 (OQ187772)).
RESULTS AND DISCUSSION
From the three M. reticulatus snakes, a total of 38 ticks were collected which consisted of 13 individuals of A. helvolum (11 males, 2 females) and 25 individuals of A. varanense (20 males, 5 females) which were confirmed by morphological examination. There were no ticks found infesting the P. bivittatus.
Amblyomma helvolum had 3/3 dentition, long and narrow palps, and oval porose areas on the rectangular basis capituli. The male ticks had an ovoid scutum with metallic and yellowish patches of ornamentation. The female ticks, on the other hand, had no scutum ornamentations. The eyes were flat and located at the lateral margins of the scutum. Coxa I bore a pair of triangular spurs, with the external spur about twice as long as internal one. Coxae II-IV each bore a single, triangular spur.
Amblyomma varanense had 3/3 dentition, long and narrow palps, and oval porose areas on the rectangular basis capituli. The male ticks had a reddish-brown round scutum, nearly as broad as long, with five metallic-green spots of variable thickness, shape, and intensity. The female ticks had a reddish-brown cordiform scutum with three greenish metallic spots. Coxa I bore two short, distinct, and separated spurs; the external spur was slightly longer than the internal. Coxae II-IV each bore a single, blunt spur about as wide as long. PCR was used to confirm the identification of ticks.
Amblyomma helvolum has a natural distribution that extends from the Nicobar Islands of India eastward through parts of Thailand, Laos, Malaysia, Singapore, Vietnam, Indonesia, the Philippines, and Taiwan ((Auffenberg, 1988);(Kolonin, 1995);
Anastos G. 1950. The Scutate ticks, or Ixodidae, of Indonesia. Entomol Am 1-4:1-144.
Anderson BE, Tzianabos T. 1989. Comparative sequence analysis of a genus-common rickettsial antigen gene. J Bacteriol 171(9):5199-201. DOI: 10.1128/jb.171.9.5199-5201.1989 DOI: https://doi.org/10.1128/jb.171.9.5199-5201.1989
Andoh M, Sakata A, Takano A, Kawabata H, Fujita H, Une Y … Ando S. 2015. Detection of Rickettsia and Ehrlichia spp. in ticks associated with exotic reptiles and amphibians imported into Japan. PLoS One 10(7):e0133700. DOI: 10.1371/journal.pone.0133700 DOI: https://doi.org/10.1371/journal.pone.0133700
Auffenberg T. 1988. Amblyomma helvolum (Acarina: Ixodidae) as a parasite of varanid and scincid reptiles in the Philippines. Int J Parasitol 18(7):937-45. DOI: 10.1016/0020-7519(88)90176-2 DOI: https://doi.org/10.1016/0020-7519(88)90176-2
Bunikis J, Barbour AG. 2005. Third Borrelia species in white-footed mice. Emerg Infect Dis 11(7):1150-1. DOI: 10.3201/eid1107.041355 DOI: https://doi.org/10.3201/eid1107.041355
Burridge MJ. 2001. Ticks (Acari: Ixodidae) spread by the international trade in reptiles and their potential roles in dissemination of diseases. Bull Entomol Res 91(1):3-23. DOI: https://doi.org/10.1079/BER200071
Chao LL, Hsieh CK, Shih CM. 2013. First report of Amblyomma helvolum (Acari: Ixodidae) from the Taiwan stink snake, Elaphe carinata (Reptilia: Colubridae), collected in southern Taiwan. Ticks Tick Borne Dis 4(3):246-50. DOI: 10.1016/j.ttbdis.2012.11.002 DOI: https://doi.org/10.1016/j.ttbdis.2012.11.002
Doornbos K, Sumrandee C, Ruang-Areerate T, Baimai V, Trinachartvanit W, Ahantarig A. 2013. Rickettsia sp. closely related to Rickettsia raoultii (Rickettsiales: Rickettsiaceae) in an Amblyomma helvolum (Acarina: Ixodidae) tick from a Varanus salvator (Squamata: Varanidae) in Thailand. J Med Entomol 50(1):217-20. DOI: 10.1603/me12010 DOI: https://doi.org/10.1603/ME12010
Estrada-Peña A, Jongejan F. 1999. Ticks feeding on humans: A review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol 23(9):685-715. DOI: 10.1023/a:1006241108739 DOI: https://doi.org/10.1023/A:1006241108739
Franke J, Hildebrandt A, Dorn W. 2013. Exploring gaps in our knowledge on Lyme borreliosis spirochaetes: Updates on complex heterogeneity, ecology, and pathogenicity. Ticks Tick Borne Dis 4(1-2):11-25. DOI: 10.1016/j.ttbdis.2012.06.007 DOI: https://doi.org/10.1016/j.ttbdis.2012.06.007
Imaoka K, Kaneko S, Tabara K, Kusatake K, Morita E. 2011. The first human case of Rickettsia tamurae infection in Japan. Case Rep Dermatol 3(1):68-73. DOI: 10.1159/000326941 DOI: https://doi.org/10.1159/000326941
Kho KL, Koh FX, Tay ST. 2015. Molecular evidence of potential novel spotted fever group rickettsiae, Anaplasma and Ehrlichia species in Amblyomma ticks parasitizing wild snakes. Parasites Vectors 8:112. DOI: 10.1186/s13071-015-0719-3 DOI: https://doi.org/10.1186/s13071-015-0719-3
Kohls GM. 1957. Malaysian parasites XVII ticks (Ixodoidea) of Borneo and Malaya. Stud Inst Med Res Malaya 28:65-94.
Kolonin GV. 1995. Review of the ixodid tick fauna (Acari: Ixodidae) of Vietnam. J Med Entomol 32:276-82. DOI: 10.1093/jmedent/32.3.276 DOI: https://doi.org/10.1093/jmedent/32.3.276
Mariana A, Vellayan S, Halimaton I, Ho TM. 2011. Acariasis on pet Burmese python, Python molurus bivittatus in Malaysia. Asian Pac J Trop Med 4(3):227-8. DOI: 10.1016/S1995-7645(11)60075-8 DOI: https://doi.org/10.1016/S1995-7645(11)60075-8
Murray-Dickson G, Ghazali M, Ogden R, Brown R, Auliya M. 2017. Phylogeography of the reticulated python (Malayopython reticulatus ssp.): Conservation implications for the worlds' most traded snake species. PLoS One. 12(8):e0182049. DOI: 10.1371/journal.pone.0182049 DOI: https://doi.org/10.1371/journal.pone.0182049
Pandit P, Bandivdekar R, Geevarghese G, Pande S, Mandke O. 2011. Tick infestation on wild snakes in northern part of western Ghats of India. J Med Entomol 48(3):504-7. DOI: 10.1603/me10164 DOI: https://doi.org/10.1603/ME10164
Petney TN, Keirans JE. 1995. Ticks of the genera Amblyomma and Hyalomma from South-East Asia. Trop Biomed 12:45-56.
Phongmany S, Rolain JM, Phetsouvanh R, Blacksell SD, Soukkhaseum V, Rasachack B … Newton PN. 2006. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis 12(2):256-62. DOI: 10.3201/eid1202.050900 DOI: https://doi.org/10.3201/eid1202.050900
Raoult D, Lakos A, Fenollar F, Beytout J, Brouqui P, Fournier PE. 2002. Spotless rickettsiosis caused by Rickettsia slovaca and associated with Dermacentor ticks. Clin Infect Dis 34(10):1331-6. DOI: 10.1086/340100 DOI: https://doi.org/10.1086/340100
Sophia HF, Supriyono, Soviana S, Novianto D, Hadi UK. 2023. Molecular detection of Borrelia spp. (Spirochaetales: Borreliaceae) in ticks (Acari: Ixodidae) collected from tortoises in Java, Indonesia. Biodiversitas 24(12): 6852-7. DOI: 10.13057/biodiv/d241246 DOI: https://doi.org/10.13057/biodiv/d241246
Sumrandee C, Hirunkanokpun S, Doornbos K, Kitthawee S, Baimai V, Grubhoffer L … Ahantarig A. 2014. Molecular detection of Rickettsia species in Amblyomma ticks collected from snakes in Thailand. Ticks Tick Borne Dis 5(6):632-40. DOI: 10.1016/j.ttbdis.2014.04.013 DOI: https://doi.org/10.1016/j.ttbdis.2014.04.013
Supriyono, Takano A, Kuwata R, Shimoda H, Hadi UK, Setiyono A … Maeda K. 2019. Detection and isolation of tick-borne bacteria (Anaplasma spp., Rickettsia spp., and Borrelia spp.) in Amblyomma varanense ticks on lizard (Varanus salvator). Microbiol Immunol 63(8):328-33. DOI: 10.1111/1348-0421.12721 DOI: https://doi.org/10.1111/1348-0421.12721
Takano A, Fujita H, Kadosaka T, Takahashi M, Yamauchi T, Ishiguro F … Kawabata H, 2014. Construction of a DNA database for ticks collected in Japan: application of molecular identification based on the mitochondrial 16S rDNA gene. Med Entomol Zool 65(1):13-21. DOI: 10.7601/mez.65.13 DOI: https://doi.org/10.7601/mez.65.13
Takano A, Goka K, Une Y, Shimada Y, Fujita H, Shiino T, Watanabe H, Kawabata H. 2010. Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ Microbiol 12(1):134-46. DOI: 10.1111/j.1462-2920.2009.02054.x DOI: https://doi.org/10.1111/j.1462-2920.2009.02054.x
Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24(8):1596-9. DOI: 10.1093/molbev/msm092 DOI: https://doi.org/10.1093/molbev/msm092
Tay ST, Ho TM, Rohani MY, Shamala D. 2000. Antibody prevalence of Orientia tsutsugamushi, Rickettsia typhi and TT118 spotted fever group rickettsiae among febrile patients in rural areas of Malaysia. Trans R Soc Trop Med Hyg 94:280-4 DOI: https://doi.org/10.1016/S0035-9203(00)90322-5
Trinachartvanit W, Hirunkanokpun S, Sudsangiem R, Lijuan W, Boonkusol D, Baimai V, Ahantarig A. 2016. Borrelia sp. phylogenetically different from Lyme disease- and relapsing fever-related Borrelia spp. in Amblyomma varanense from Python reticulatus. Parasites Vectors 9:359. DOI: 10.1186/s13071-016-1629-8 DOI: https://doi.org/10.1186/s13071-016-1629-8
Copyright (c) 2025 Supriyono, Hana Faizah Sophia, Upik Kesumawati Hadi, Susi Soviana

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish with this journal agree with the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work 1 year after publication simultaneously licensed under a Creative Commons attribution-noncommerical-noderivates 4.0 International License that allows others to share, copy and redistribute the work in any medium or format, but only where the use is for non-commercial purposes and an acknowledgement of the work's authorship and initial publication in this journal is mentioned.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).


