INTRODUCTION
The feed factor is an essential element of a ruminant fattening strategy. The availability and synergistic interaction of nutrients in the diet are crucial factors in determining the productivity of animals. Alternative feed sources are necessary due to the typically expensive cost commercial feed and the intermittent availability of protein feed. Consequently, meeting the nutritional requirements of cattle and maintaining a consistent feed supply can be labor-intensive. Agro-industrial waste can serve as a potential substitute for cattle feed due to its retention of essential nutrients and consistent availability. The addition of agro-industrial wastes, such as carica processed waste, to complete feed is expected to minimize feed costs, reduce waste, and increase small-scale animal production (Lalramhlimi et al. 2022). One of the initiatives to build a low-cost and effective feed business is using locally accessible agricultural and plantation wastes in each region, along with reducing agro-industrial waste. Despite the extensive utilization of the country's abundant feed ingredients, the need for these feeds remains unfulfilled (Kim et al. 2019). Therefore, further research is required to investigate unconventional materials. Carica Dieng confections have become into basic commodities, possessing economic significance. Annually, a total of 1100-1200 tons of fruit is harvested, with the involvement of 30 middle, small, and micro businesses (Ningsih et al. 2019).
The Carica seeds and their membranes are remnants resulting from the preparation of the carica fruit. According to Briones-Labarca et al. (2015), the ripe seeds have a moisture content of 3.50%, an ash content of 3.96%, a crude protein content of 31.84%, and a crude fiber content of 24.41%. The relatively high protein content of Carica Dieng seeds might provide some advantage. Multiple research studies have proven that Dieng carica seeds contain anti-nutritional factor tannins and saponins (Kiranmayi 2014); hence, the utilization of Dieng carica seeds as feed needs further research. Natural foods and feedstuffs contain chemical substances known as anti-nutritional factors (ANFs) which are generated through the metabolic processes of species and various factors that hinder optimal nutrition. These factors include the inactivation of particular nutrients, a deceleration of the digestive system, and a reduction in the metabolic utilization of the food or feed (Thakur et al. 2017). Tannins are polyphenolic chemicals classified into two types depending on their chemical structure: condensed and hydrolyzable tannins. Green plants typically produce different quantities of tannins, resulting in different biological properties. The presence of many phenolic groups accounts for tannins' strong affinity for proteins. These provide numerous opportunities for peptide carbonyl groups to bind. The creation of such complexes is specific regarding the tannin and protein involved, with the degree of affinity between the molecules in each chemical property (Bunglavan and Dutta 2013). According to Trisnadewi et al. (2014), an increased concentration of tannins attaches to carbohydrates and proteins, thereby impeding the ability of rumen microorganisms to degrade these substances and consequently diminishing enzyme activity. A reduction in the population of rumen bacteria will correspondingly lead to impaired nutrient assimilation and decomposition, as well as reduced access to carbohydrates and proteins within the rumen.
Saponin is a glycoside that has an aglycone in the form of sapogenin. The chemical structure of saponins is in the form of a glycoside consisting of glycone and aglycones. The glycone part is a sugar group such as glucose, fructose, and other types of sugar, while the aglycone part is a sapogenin (Nurzaman et al. 2018). Saponins are glycosides with aglycones in the form of steroids and triterpenoids (Yanuartono et al. 2017). Steroid saponins are often found in single-seeded or monocot plants (Negi et al. 2013), and triterpenoid saponins are often found in double-seeded plants or dicots (Yanuartono et al. 2017). Anti-nutritional factors (ANFs), which are naturally present compounds in plants, have the potential to adversely affect the value of feed when administered to livestock in specific amounts. They can affect biological aspects, disrupt body metabolism, reduce livestock productivity, and inhibit livestock growth and health (Jayanegara 2018; Murni et al. 2012). Because ANFs can stunt the growth of livestock that consume them, their presence in feed ingredients may be a ration-limiting factor. It is necessary to study the ANFs content in Carica Dieng seeds. This step is critical to minimize the various adverse effects of ANFs. Boiling has been identified as a potential technique for diminishing the antinutrient content (Ndidi et al. 2014). In their research, Assam et al. (2019) demonstrate that water steaming can also reduce the content of tannins, HCN, saponins, and phytate.
The ability of ruminant livestock to utilize feed containing antinutrients varies. The tolerance of sheep to the antinutrients in boiled carica seeds has yet to be discovered, thus requiring research on the levels of carica seeds in sheep rations. The addition of boiled carica seeds at different levels aims to determine the maximum limit of antinutrients that sheep can tolerate. With this knowledge, maximizing the use of carica seeds, which are processed by boiling as a source of protein in sheep, can be used as safe feed for their productivity. It is necessary to do in vitro testing on boiled carica seeds to assess the nutrient quality, specifically the digestibility of dry matter and organic matter in sheep rations. It can be achieved by replicating the physiological processes that occur in the digestive system of livestock.
MATERIALS AND METHODS
Time and Location of Research
The Dieng carica seeds used in this study were collected from the carica beverage manufacturing company Dieng, Banjarnegara, Central Java, Indonesia. The collection of seeds was conducted in April 2022 (Figure 1). The Carica Dieng fruit and its seeds were used as samples in this study (Figure 2).
Procedures
The carica seeds were separated from the carica fruit through peeling and removal. The seeds underwent processing in accordance with the procedure outlined by Talabi et al. (2016). The boiling process lasted for durations of 0, 10, and 20 minutes. In each case, the water was heated until it reached the boiling point prior to pouring the seeds, and they were then left to boil for the duration length. Subsequently, the seeds were drained of water, dried, and boiled. They were then examined to determine the levels of tannins, saponins, amino acids, as well as the digestibility of dry and organic matter using in vitro analysis.
Nutritional Component Analysis
Chemical analysis of carica seed samples that had been boiled included proximate analysis: dry matter (DM), ash, organic matter (OM), crude protein (CP), ether extract (EE), and crude fiber (CF), according to AOAC (2005); Amino acid analysis In house method (ICI Instrument Method 1988), tannin content (Folin Ciocalteau method (Chaovanalikit and Wrolstad, 2004), saponins (Pasaribu et al. 2014), dry and organic matter digestibility was determined using the Tilley and Terry method (1963).

a

c
Experimental Design and Data Analysis
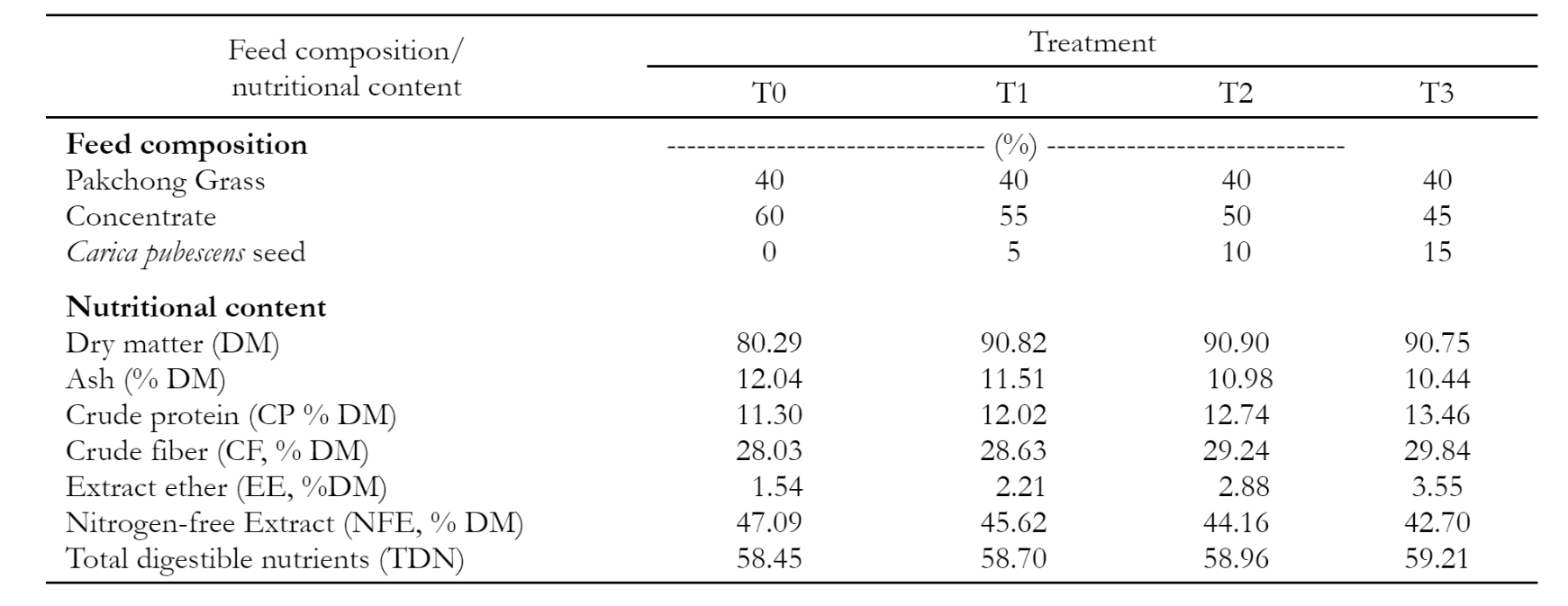
Carica Dieng seeds used in this research were collected from Dieng, Central Java, Indonesia. As part of the preliminary investigation, the experiment examined the existence of tannins and saponins and assessed the reduction of their levels by a physical treatment (boiling). This research used a completely randomized design consisting of three treatments: T0 without boiling, T1 = 10 minutes, T2 = 20 minutes, and six replications. The Carica seeds were subjected to boiling water for an appropriate duration during their processing. Their amino acid composition and the best results were carefully examined and discussed descriptively. Carica seeds were added to complete feed and tested in vitro to evaluate the digestibility of dry matter and organic matter. Testing was done using a completely randomized design with four treatments and five replications. Carica seeds were added to complete feed, consisting of T0 = 40% Pak Chong grass + 60% concentrate + 0% addition of carica seeds, T1 = 40% Pak Chong grass + 55% concentrate + addition of 5% carica seeds, T2 = 40% Pakchong grass + 50% Concentrate+ addition of 10% carica seeds, T3 = 40% Pakchong Grass+45% Concentrate+ addition of 15% carica seeds (Table 1). Data on the presence of tannins and saponins and their digestibility were analyzed using analysis of variance (ANOVA) at the 5% level. If there were differences between treatments, continued further testing with the Duncan Multiple Range Test (DMRT).
Experimental Design and Data Analysis
Carica Dieng seeds used in this research were collected from Dieng, Central Java, Indonesia. As part of the preliminary investigation, the experiment examined the existence of tannins and saponins and assessed the reduction of their levels by a physical treatment (boiling). This research used a completely randomized design consisting of three treatments: T0 without boiling, T1 = 10 minutes, T2 = 20 minutes, and six replications. The Carica seeds were subjected to boiling water for an appropriate duration during their processing. Their amino acid composition and the best results were carefully examined and discussed descriptively. Carica seeds were added to complete feed and tested in vitro to evaluate the digestibility of dry matter and organic matter. Testing was done using a completely randomized design with four treatments and five replications. Carica seeds were added to complete feed, consisting of T0 = 40% Pak Chong grass + 60% concentrate + 0% addition of carica seeds, T1 = 40% Pak Chong grass + 55% concentrate + addition of 5% carica seeds, T2 = 40% Pakchong grass + 50% Concentrate+ addition of 10% carica seeds, T3 = 40% Pakchong Grass+45% Concentrate+ addition of 15% carica seeds (Table 1). Data on the presence of tannins and saponins and their digestibility were analyzed using analysis of variance (ANOVA) at the 5% level. If there were differences between treatments, continued further testing with the Duncan Multiple Range Test (DMRT).
RESULTS AND DISCUSSION
Effect of Processing Antinutritional (Tannin, Saponin) Content
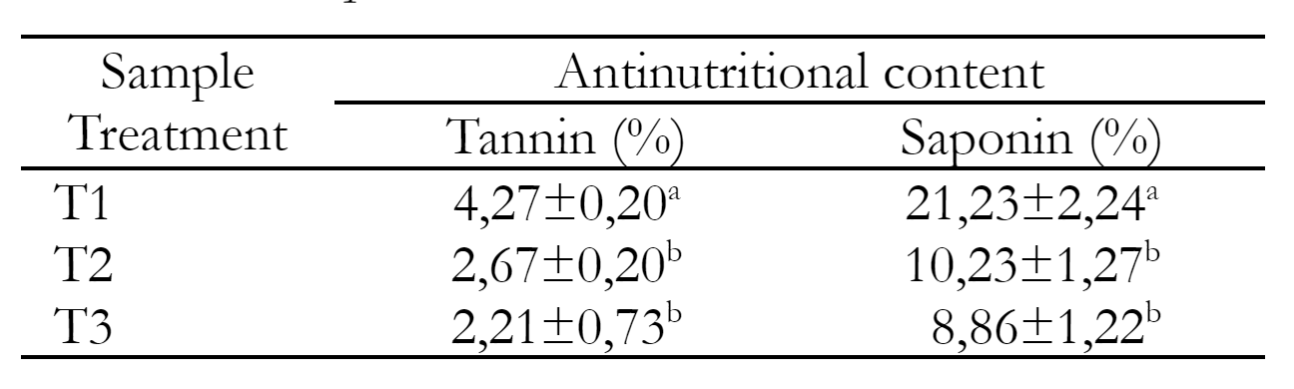
This research shows that there is an influence between the boiling process on reducing the tannin and saponin levels of Carica Dieng seeds (p<0.05), where the boiling treatment at T2 and T3 significantly decreased compared to T1, but T2 and T3 were relatively the same. Anti-nutritional quality of Carica pubescens seeds with prolonged boiling treatment is shown in Table 2. The tannin content of the T2 treatment (boiling for 10 minutes) was 2.67% lower than the T1 treatment (raw materials without boiling). The tannin concentration decreased to (2.21%) under T3 treatment (20 minutes of boiling). This study relied on the research conducted by Jamarun et al. (2021), who find that mangrove tannin levels fall by 7.4% after 10 minutes of boiling. Assam et al. (2019) report that boiling for up to 30 minutes reduce the tannin and saponin content in Cassia tora seeds. This drop happened as a result of the lengthy boiling time, which might cause the connections between the fibers in carica seeds to loosen. Due to the osmosis process, which pushes tannins out of the leaves and causes them to dissolve in alkaline water through diffusion activity, the tannin content will be reduced (Perdana et al. 2012). Acids, bases, and enzymes can hydrolyze tannin. Tannins will break down into glucose and gallic acid if heated at 98.89ºC - 101.67ºC (Muhammad et al. 2015). Tannins are readily soluble in water, and solubility increases when dissolved in hot water (Perdana et al. 2012). According to the theory that heat denatures proteins and because some antinutritional components present in carica seeds are protein compounds, heat tends to denature them, the reduction in antinutrient levels caused by boiling in this study. While tannins discolor seeds and bind protein through hydrogen bonds and hydrophobic interactions.
Table 2 indicates that the saponin content of Carica pubescens seeds decreased from 21.23% to 8.86%. This result is consistent with a previous study by Lakram et al. (2018). They report that boiling for 25 minutes at 100 ˚C significantly reduce saponin levels. The reduction in saponin content can be attributed to the thermal degradation associated with the heat supplied to the cooked plants. The decrease was also observed by Hemmige et al. (2017) and Ilelaboye et al. (2013). Saponins are water-soluble chemicals that are highly soluble in cold and hot water (Chairunnisa et al. 2019).
Saponin is a complex glycoside that is found in plants, known for its foaming properties. Therefore, it will produce foam when it reacts with water and is shaken. Boiling may also remove components from the material that are deemed superfluous, particularly those that are water-soluble. Temitope et al. (2013) identify that heating can reduce saponin concentrations in various plant species. According to Chaturvedi et al. (2012), boiling can lower the amount of saponin in soybeans (Glycine max Linn). Saponins and plants with high saponin content harm protozoa by building an irreversible combination with steroids in the protozoan cells (Yanuartono et al. 2017). This decrease in the protozoa population in the rumen is likely to have several beneficial effects, including improved nitrogen metabolism efficiency, decreased methane gas emissions, changes in the population of bacteria and fungi in the rumen, and the potential for increased flow of bacterial protein to the lower digestive tract. The findings of this study were corroborated by Wang et al. (2012), who claimed that adding saponins from tea to the diet might suppress methanogenesis. This reduction is expected to have positive effects on the environment and enhance the productivity of animal rearing for feed purposes.
Chemical Composition
The results of boiling the seeds with the time of 10 minutes were tested descriptively for their nutrient content and amino acids. The proximate analysis showed that carica seeds contained 12.75% Moisture, 6.57% Ash, 14.85% Fat, 27.98% Crude Protein, and 29.89% Crude Fiber, 35.32% Nitrogen Free Extract (Table 3). More than 20% of the protein in Carica pubescens seeds makes them an excellent source of protein for rations. High molecular weight peptides make up protein, a chemical material with various bodily functions. The primary building block of muscle tissue is protein.
The amount of microbial protein supply, which is dependent on the availability of carbohydrates and nitrogen (not always in the form of protein), as well as the amount of protein that is freely degraded in the rumen, is the primary determinant of the amount of essential and non-essential amino acids that are available to tissues in ruminants (Wu et al. 2014). The optimal protein requirement for ruminants is determined by their capacity to digest protein. Concentrate feed containing a variety of proteins can provide a more cost-effective source of protein. It is because the combination of proteins with multiple types and amounts of amino acids increases the ability of rumen microbes to rapidly synthesize all the necessary amino acids (Williamson and Payne, 1978; Pathak, 2008).
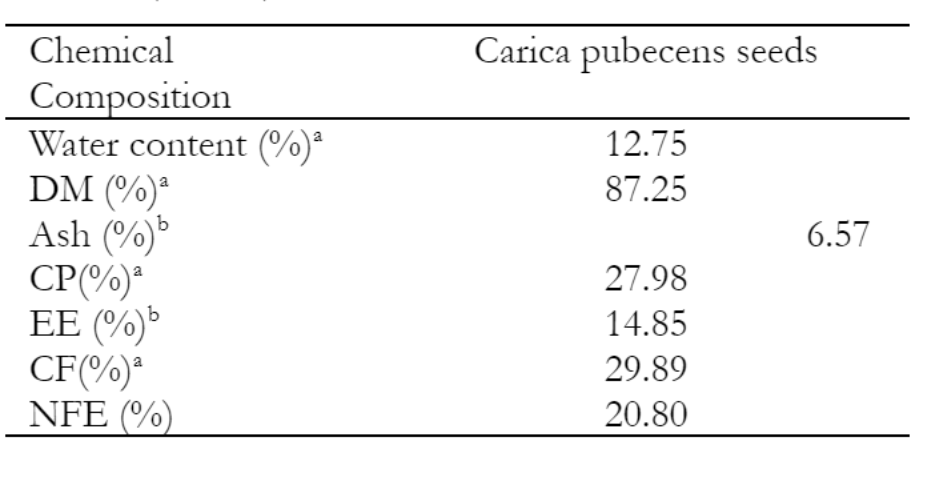
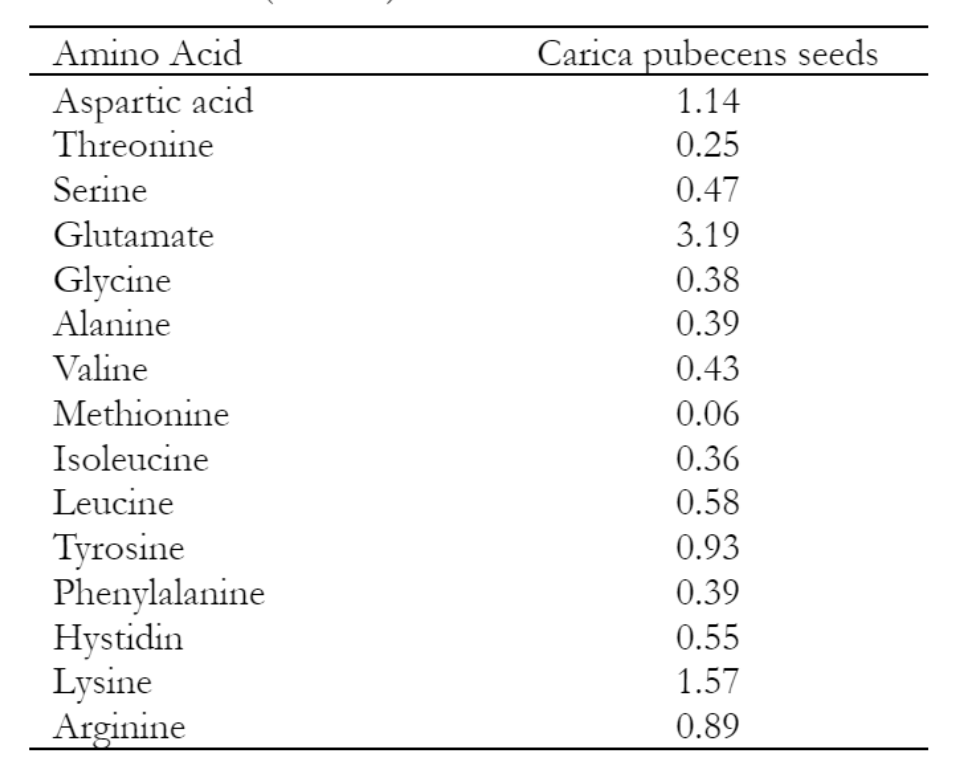
Table 4 presents the results of the analysis of amino acids from the seeds of Carica pubescens. Carica pubescens seed essential amino acids are dominated by lysine (1.57% w/w), and non-essential amino acids by glutamic acid (3.19% w/w). Amino acids, which are found in proteins, serve the purpose of constructing new tissues, facilitating energy metabolism, synthesizing hormones, and acting as essential catalysts for several physiological processes in the body (Li et al. 2020).
Carica pubescens seeds have a relatively high protein content, which is considered important as a new source of nutrition for ruminants. The protein content in Carica pubescens seeds is around 24%, equivalent to that of coconut meal. Amino acid analysis using HPLC showed that the seeds of Carica pubecens contained essential and non-essential amino acids (Table 2). The highest essential amino acid from Carica pubescens seeds is dominated by lysine (1.57% w/w). Lysine is necessary because it is one of the precursors to the growth of rumen microorganisms (Kong et al. 2021). Lysine has a part in forming carnitine, which can stimulate growth, guard against ammonia toxicity, and improve the body's ability to fend off extreme temperature fluctuations (Aristasari et al. 2018). Lysine inclusion in the meal can promote increased protein synthesis. Feed efficiency and feed consumption can both be improved by lysine content (Aristasari et al. 2018). Lysine tends to boost the feed's protein, fat, and calorie content when added to the ration. The energy content of the meal can also determine the level of feed efficiency. Beyond that, Lysine can improve a feed's ability to be digested by the ileum (intestine). Livestock may be absorbed fast to increase feed efficiency and give livestock a feeling of fullness and strong growth rates. The amino acid tyrosine, which regulates the body's reaction to stress, can be more easily digested after lysine has been added to feed increases the anti-inflammatory action and aids in the healing and prevention of ulcerative colitis and peptic ulcers (Aristasari et al. 2018).
The highest non-essential amino acids from Carica pubescens seeds were glutamic acid content (3.19% w/w). The creation of proteins depends on glutamic acid, which also serves as a source of energy for the intestinal lining cells. Enhances immunological response. Glutamic acid is a non-essential amino acid that functions as a building block for protein, as a precursor for several non-essential amino acids, and helps the body's metabolism (Slyamova et al. 2016) as well as a neurotransmitter for taste (Huang et al. 2019). Glutamic acid also functions as an antioxidant regulating inducible nitric oxide synthase (iNOS) as protection against intracellular parasites, bacteria, fungi, viruses, and protozoa (Maslami et al. 2018). Glutamic acid is an alternative feed additive that is safe and environmentally friendly in improving carcass performance and quality (Maslami et al. 2018).
In-vitro dry matter and organic matter digestibility
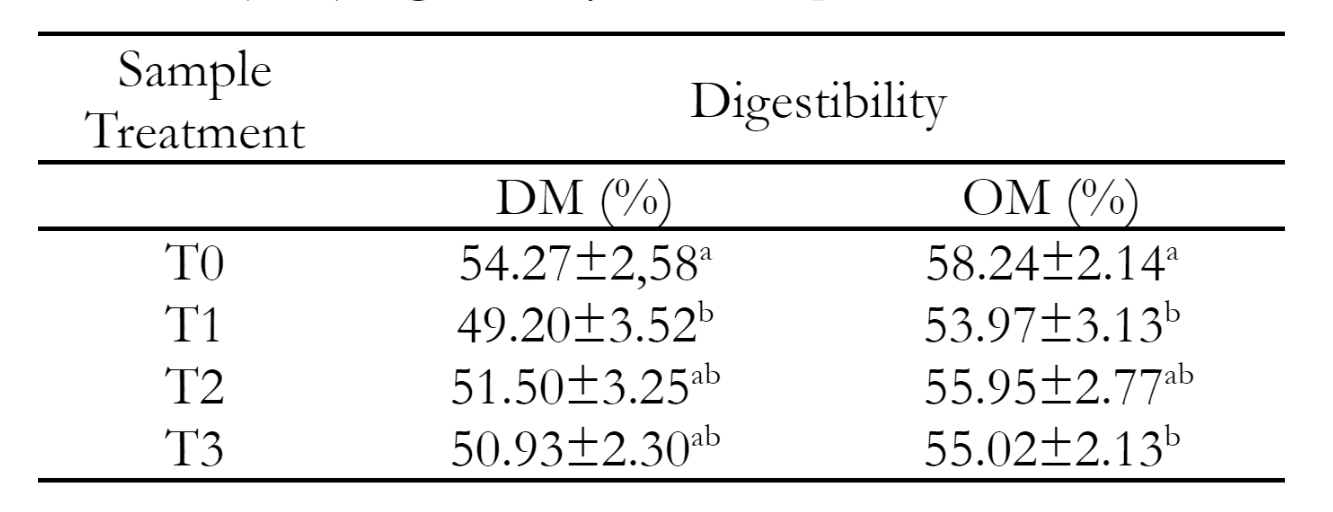
Digestibility is an initial sign of the availability of different nutrients in a food source that livestock will eat. The high nutritional content consumed post-digestion indicates that the meal is easily digestible (Mayulu et al. 2019). Table 5 shows the digestibility of dry and organic matter with the addition of carica seed content. The T0 treatment, which did not involve the addition of carica seeds, showed no significant difference compared to the treatments with 10% and 15% carica seeds. However, it exhibited a major difference in dry matter digestibility compared to the treatment with 5% carica seeds. The study found that the lowest digestibility was observed when employing a 5% concentration of Carica. This is believed to be due to the varying levels of Carica pubescens seeds used in the study, which resulted in diverse digestibility outcomes. Carica levels cause different nutrient content, such as the amount of crude protein, crude fiber, and tannins in the treated feed. This decrease in digestibility is likely caused by increased crude fiber content due to adding Carica pubescens seeds to complete feed. Crude fiber is a component of organic material that is difficult to digest in rumen. Increasing the crude fiber content will reduce the digestibility of dry matter, crude protein and energy. Setiasih et al. (2023) explained that in their research, the more crude fiber in the feed material, the thicker the cell walls and the more resistant it is to fiber-digesting microorganisms, causing the digestibility of the material to be lower. Rumen microorganisms participate in the fermentation process. Feed intake depends on activity, and the nutrients contained in feed ingredients impact rumen microbial activity (Sandi et al. 2015). An increase or decrease in digestibility is shown in the number and activity of microbes (Mayulu 2014). The presence of tannins in carica seeds is thought to reduce the digestibility of feed ingredients. This decrease was due to tannin being able to bind protein so that rumen microbes could not degrade it. However, if the tannin concentration is not well controlled, the presence of tannins in protein protection will enhance the availability of high-quality feed protein. This can lead to disruptions in overall digestibility and nutrient absorption, since tannins interact with protein, fiber, vitamins, and minerals. In addition, Mertens & Grant (2020) state that the physical composition of feed ingredients and their ratios vary. Composition, temperature and speed through the digestive tract are some variables that influence the digestibility of dry and organic matter.
The study examined the impact of Carica seed content on the digestibility of organic matter. The treatment without any carica seed addition showed no significant difference compared to the treatment with 10% carica seed. However, it did show a significant difference when compared to the treatments with 5% and 15% carica seed content. The digestibility of organic matter describes the availability of nutrients from feed. The livestock digestive system can digest organic materials, which include food in the form of organic material components, including carbohydrates, proteins, lipids and vitamins. In order to make organic components in feed more accessible, it is essential to convert them from insoluble to soluble form (Suardin et al. 2014). Ismail (2023) assert that the digestibility of organic matter is closely related to the digestibility of dry matter because some dry matter consists of organic matter. Factors that influence the digestibility of organic materials are the crude fiber. The increasing crude fiber content in feed tends to increase the cellulose, hemicellulose and lignin content, is caused by microbes being unable to optimally digest the crude fiber components contained in feed ingredients resulting in lower organic matter digestibility values (Tafsin 2019).
CONCLUSION
The research findings indicated a significant decrease in the antinutrient content (tannins and saponins) of boiling Carica pubescens seeds. The inclusion of Carica seed levels in complete feed ingredients can meet the protein requirements as they have a high protein content. Adding 10-15% of Carica seed levels does not negatively affect in vitro digestibility.
ACKNOWLEDGMENTS
This work was partially supported by Puslapdik Kemdikbud (Center for Education Financial Services, Ministry of Education, Cultural Research and Technology, LPDP (Indonesian Education Endowment Fund) to fund this research through the Indonesian Education Scholarship (BPI) or the Indonesian Education Scholarship Program, the Faculty of Animal Husbandry and Agriculture at Diponegoro University, and the Faculty of Agriculture at Tidar University.
REFERENCES
- AOAC. 2005. Official methods of analysis (17th ed.). Washington, DC: Association of Official Analytical Chemistry.
- Aristasari E, Nur‘Aini, RA., Nopita, W, Lamid, M, Al-Arif, MA. 2020. The growth, protein content, and fatty acid of catfish meat (Pangasius sp.) With the addition of different lysine doses in commercial feed. In IOP Conference Series: Earth and Environmental Science (Vol. 441, No. 1, p. 012018). IOP Publishing.
- Assam ED, Ndelekwute, EK, Demo, K, Okonkwo, AC, Ukachukwu, SN. 2019. Effect of boiling on biochemical composition of raw Cassia Tora seeds and its potential as feed ingredient in poultry feeds. Nigerian Journal of Animal Science, 21(3): 288-293.
- Briones-Labarca V, Plaza-Morales M, Giovagnoli-Vicuña C, Jamett F. 2015. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT-Food Science and Technology, 60(1): 525-534.
- Bunglavan SJ, Dutta N. 2013. Use of tannins as organic protectants of protein in the digestion of ruminants. J. Livestock Sci. 4: 67-77.
- Chairunnisa S, Wartini, NM, Suhendra, L. 2019. Pengaruh Suhu dan Waktu Maserasi terhadap Karakteristik Ekstrak Daun Bidara (Ziziphus mauritiana L.) sebagai Sumber Saponin J. Rekayasa Dan Manaj. Agroindustri 7: 551.
- Chaovanalikit A, Wrolstad RE. 2004. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. Journal of food science 69(1): FCT67-FCT72.
- Chaturvedi S, Hemamalini R, Sunil KK. 2012. Effect of processing conditions on saponin content and antioxidant activity of Indian varieties of soybean (Glycine max Linn.). Annals of Phytomedicine. 1(1): 62-68.
- Hemmige NH., Abbey L, Asiedu SK. 2017. An overview of nutritional and antinutritional factors in green leafy vegetables. Int J Hortic Sci. 1(2):0001 https://doi.org/10.15406/hij.2017.01.00011
- Huang Y, Duan W, Wang L, Xiao J, Zhang Y. 2019. Orthogonal optimization of beef stir-fried process followed by isolation and identification of the umami peptides by consecutive chromatography and LC-Q-TOF/MS.Int J Food Prop. 22(1): 1773-1785.
- Ilelaboye NOA, Amoo, IA, Pikuda, OO. 2013. Effect of cooking methods on mineral and anti nutrient composition of some green leafy vegetables. Archives of Applied Science Research, 5(3): 254-260.
- Ismail N, Obeidat BS. 2023. Olive leaves as alternative feed for finishing lambs: evaluation of feed intake, nutrients digestibility, growth performance, and carcase quality. Italian Journal of Animal Science, 22(1): 214-221.
- Jamarun N, Pazla R, Yanti G. 2021. Effect of boiling on in-vitro nutrients digestibility, rumen fluid characteristics, and tannin content of mangrove (Avicennia marina) leaves as animal feed. In IOP conference series: earth and environmental science (Vol. 733, No. 1, p. 012106). IOP Publishing.
- Jayanegara A, Harahap RP, Ridla M, Laconi EB, Nahrowi. 2018. Chemical composition and methane emission of some tropical forage legumes from Indonesia. AIP Conference Proceedings 2021, 050002.
- Kim, SW., Less JF, Wang L, Yan T, Kiron V, Kaushik SJ, Lei XG. 2019. Meeting global feed protein demand: challenge, opportunity, and strategy. Annual review of animal biosciences, 7: 221-243.Kiranmayi P 2014. Bioactive compounds in plants act as anti-nutritional factors. International Journal of Current Pharmaceutical Research 6(2): 36-38.
- Kong F, Li Y, Diao Q, Bi Y, Tu Y. 2021. The crucial role of lysine in the hepatic metabolism of growing Holstein dairy heifers as revealed by LC-MS-based untargeted metabolomics. Animal Nutrition, 7(4), 1152-1161.
- Lakram N, En-Nahli Y, Zouhair FZ, Moutik S, Kabbour R, El Maadoudi, E. H., ... & Naciri, M. 2019. The impact of optimizing the detoxification of Argane (Argania Spinosa) press cake on nutritional quality and saponin levels. Iranian Journal of Applied Animal Science, 9(2), 235-246.
- Lalramhlimi, B, Mukherjee D, Chakraborty I, Ghosh N, Chattopadhyay A, Dey RC. 2022. Fruit and Vegetable Wastes as Livestock Feeds. In Fruits and Vegetable Wastes: Valorization to Bioproducts and Platform Chemicals: Springer Nature Singapore. p. 139-168.
- Li X, Zheng S, Wu G. 2020. Amino acid metabolism in the kidneys: nutritional and physiological significance. Amino Acids in Nutrition and Health: Amino acids in systems function and health, 71-95.
- Maslami V, Marlida Y, Nur YS, Adzitey F, Huda N. 2018. A review on potential of glutamate producing lactic acid bacteria of West Sumatera's fermented food origin, as feed additive for broiler chicken. Journal of World's Poultry Research, 8(4): 120-126.
- Mertens DR., & Grant, RJ. 2020. Digestibility and intake. Forages: the science of grassland agriculture, 2, 609-631
- Mayulu H. 2014.The nutrient digestibility of locally sheep fed with amofer palm oil byproduct-based complete feed. Internat. J. Sci. Eng 7 (2): 106-111.
- Mayulu H, Fauziah, N, Christiyanto, M, Sunarso, S, Haris MI. 2019. Digestibility value and fermentation level of local feed-based ration for sheep. Animal Production, 20(2): 95-102.
- Muhammad PH, Luh Putu, W, AAM DA. (2015). Pengaruh suhu dan lama curing terhadap kandungan senyawa bioaktif ekstrak etanol bunga kecombrang (Nicolaia speciosa Horan). Jurnal Rekayasa dan Manajemen Agroindustri, 3(4), 92-102.
- Murni R, Akmal A, Okrisandi Y. 2012. Pemanfaatan kulit buah kakao yang difermentasi dengan kapang Phanerochaeta chrysosporium sebagai pengganti hijauan dalam ransum ternak kambing. Agrinak. 2: 6-10.
- Ndidi US, Ndidi, CU, Olagunju, A, Muhammad A, Billy FG, Okpe O. 2014. Proximate, antinutrients and mineral composition of raw and processed (Boiled and Roasted) Sphenostylis stenocarpa seeds from Southern Kaduna, Northwest Nigeria. International Scholarly Research Notices.
- Negi JS, Negi PS, Pant GJ, Rawat MS, Negi SK. 2013. Naturally occurring saponins: Chemistry and biology. Journal of Poisonous and Medicinal Plant Research. 1(1): 001-006.
- Ningsih AS, Waspiah, W, Salsabilla, S. 2019. Indikasi Geografis atas Carica Dieng Sebagai Strategi Penguatan Ekonomi Daerah. Jurnal Suara Hukum, 1(1):105-120.
- Nurzaman F, Djajadisastra J dan Elya B, 2018. Identifikasi kandungan saponin dalam ekstrak kamboja merah (Plumeria rubra l.) dan daya surfaktan dalam sediaan kosmetik. Jurnal Kefarmasian Indonesia, 8(2): 85-93.
- Pasaribu T, Astuti DA, Wina E, Sumiati, Setiyono A. 2014a. Saponin content of Sapindus rarak pericarp affected by particle size and type of solvent, its biological activity on Eimeria tenella oocysts. Int J Poult Sci. 13: 347-352.
- Pathak AK. 2008. Various factors affecting microbial protein synthesis in the rumen. Veterinary World, 1(6): 186.
- Perdana SY, S Nirwani, Supriyantini E. 2012. The effect of ash content during boiling and soaking time of water on tannin levels of fruit and mangrove flour (Avicennia marina) J. Mar. Res. 1226-234.
- Sandi S, Ali AIM, Akbar AA. 2015. In vitro test of complete wafer ration with different adhesives. Jurnal Peternakan Sriwijaya (J. of Sriwijaya Animal Science). 4(2): 7-16.
- Slyamova AY, Sarsembayeva NB, Ussenbayev AE and Paritova AY. 2016. Influence of functional feed additive at the basis of the chankanay deposit’s zeolite to the intestinal microbiocenosis of broiler chickens. International Journal of Advances in Chemical Engineering and Biological Sciences, 3 (1): 85-87. DOI: 10.15242/IJACEBS. AE0416119.
- Suardin N, Sadiah, Aka R. 2014. Kecernaan bahan kering dan bahan organik campuran campuran rumput mulato (Brachiria hybrid.cv mulato) dengan jenis legum berbeda menggunakan cairan rumensapi. Jitro Vol 1(1): 16-22.
- Talabi JY, Osukoya OA, Ajayi OO, Adegoke GO. 2016. Nutritional and antinutritional compositions of processed Avocado (Persea americana Mill) seeds. Asian Journal of Plant Science and Research, 6(2), 6-12.
- Tafsin M. 2019. Effect of bio activator use on corn cobs as a complete feed on performance and digestibility of local sheep. In IOP Conference Series: Earth and Environmental Science (Vol. 260, No. 1, p. 012047). IOP Publishing.
- Temitope OK, Adeleke A, Joseph KH, Salau APO, Adewale B. 2013. Changes in Saponins content of some selected Nigerian vegetables during blanching and juicing. Journal of Environmental Science, Toxicology and Food Technology. 3(3): 38-42.
- Thakur NS, Kumar P. 2017. Anti-nutritional factors, their adverse effects and need for adequate processing to reduce them in food. Agric INTERNATIONAL, 4(1), 56-60.
- Tilley JMA, Terry DR. 1963. A two‐stage technique for the in vitro digestion of forage crops. Grass and forage science, 18(2), 104-111.
- Trisnadewi AAS, Cakra IGLO, Wirawan IW, Mudita IM, Sumardani NLG. 2014. Substitution of Gamal (Gliricidia sepium) with Kaliandra (Calliandra calothyrsus) in the Ration Against Invitro Digestibility. Pastura 3(2): 106-109.
- Yanuartono Y, Purnamaningsih H, Nururrozi A, Indrajulianto S. 2017. Saponin: dampak terhadap ternak (Ulasan). Jurnal Peternakan Sriwijaya, 6(2): 79-90.
- Yanuartono Y, Nururrozi A, Indarjulianto S, Purnamaningsih H. 2019. Peran protozoa pada pencernaan ruminansia dan dampak terhadap lingkungan. Ternak Tropika : Journal of Tropical Animal Production, 20(1): 16-28.
- Wang JK, Ye JA, Liu JX. 2012. Effects of tea saponins on rumen microbiota, rumen fermentation, methane production and growth performance—a review. Trop Anim Health Prod. 44: 697-706.
- Williamson G, & Payne WJA. 1978. An introduction to animal husbandry in the tropics (No. Ed. 3). Longman.
- Wu G, Bazer FW, Dai Z, Li D, Wang J ,Wu Z. 2014. Amino acid nutrition in animals: protein synthesis and beyond. Annu. Rev. Anim. Biosci., 2(1),387-417.