INTRODUCTION
Three colored langurs locally known as Lutung sentarum (Presbytis chrysomelas ssp cruciger Thomas 1892) and referenced as langur for this paper is one of the endemic primates of Bornean Island and Danau Sentarum National Park, a place used as their natural habitat. Nevertheless, it has been categorized as critically endangered species by the International Union for Conservation of Nature (IUCN), however it is yet to be registered as the protected species in accordance with the regulation of animal protection in Indonesia. This can be attributed to the absence of adequate ecological information on the species, namely, habitat characteristics and daily activities. So, far there has been no research that specifically examines the ecology of the feed and space utilization for the langur habitat. The distribution of P c ssp cruciger was found at Sungai Pelaik sub-Village but only with encounters notes, and do not provide comprehensive information on the ecology (Rifki et al. 2019). Research that has been done previously is still very finite on topics regarding habitat characteristics (Musyafa and Santoso 2020) and population estimates that still need to be repeated (Aripin et al. 2019). Sources indicate that this species are also inhabitants of the North Borneo Island additionally known in Sabah, Sarawak (Malaysia) and Brunei Darussalam, however there is not adequate information available to understand the comprehensive ecology of this species in their natural habitat (Nijman 2020).
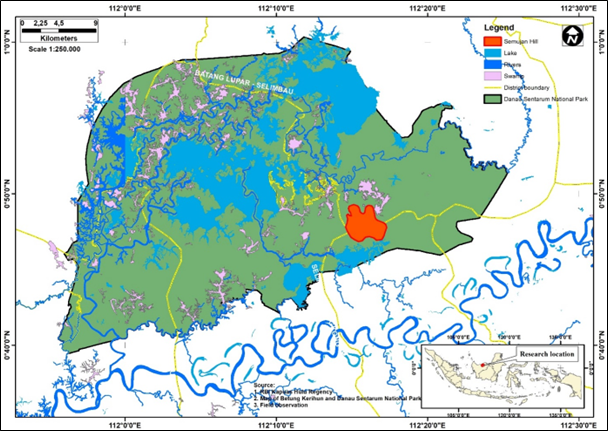
Areas that are practiced as natural habitat of langur in Danau Sentarum National Park can be found in the forest of Bukit Semujan. Administratively, these habitats scope at Lupak Mawang Resort and at government administration including the Selimbau and Jongkong District, Kapuas Hulu Regency. The condition of feed resources in the habitat of the three colored langur also affects their daily activity pattern. Habitat impacted the canopy utilization for distinct activities too, namely, moving, foraging, and social recreations (Watanabe 1981; Hadi et al. 2012). Primates such as three colored langurs usually exhibit further characteristics of their activity with vocalization or agonism from intraspecies interactions (Singh et al. 2011; Houle et al. 2006). This study aims to identify ecological characteristics in the form of habitat and feed, as well as daily activity, and ranging patterns of three colored langurs.
MATERIALS AND METHODS
Study Site and Time
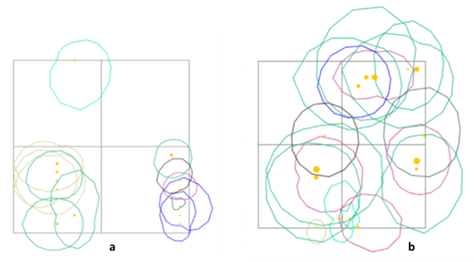
This research was conducted at Bukit Semujan, Lupak Mawang Resort, Danau Sentarum National Park, West Kalimantan, Indonesia with geographic coordinates of 00°45'–01°02' N and 111°55'–112°26' E. Primary data collected namely, group size, habitat characteristic, feeds, and daily activity were observed between July and August of 2021. Bukit Semujan has topography that ranges from flat (elevation of 60 above sea level) and continues to be wavy at an elevation of 60–80 and high cliff at an elevation of 80–300 above sea level. Land cover condition in accordance with the on Ground Truthing and Rupa Bumi Indonesia classification of Kapuas Hulu regency encompasses the primary forest at the top of the hills and mixed forest that includes secondary forest surrounding the sub-hills, swamp forest, and small area of the cultivation land. Following figures exhibit the study area (Figure 1a and 1b).

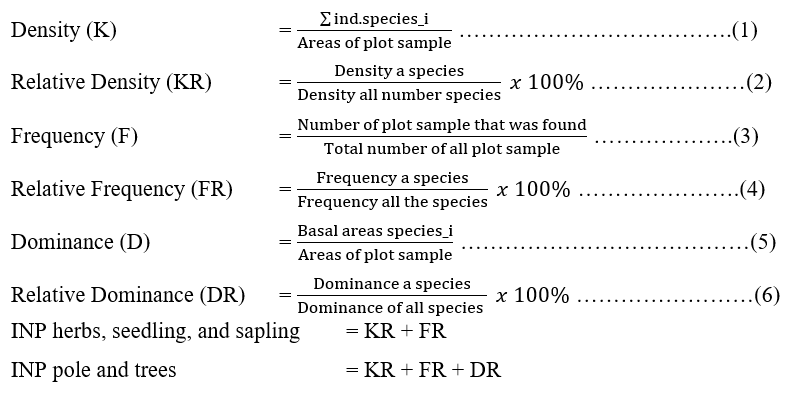
Field data of habitat characteristic implemented the plot sample with purposive sampling for every single land coverage and ecotone areas (Soerianegara and Indrawan 1988). Total sampling comprised of 12 plots, located in swamp forest, secondary, and mixed forest. Plot sample size were prepared in blocks of 20 x 20 m for trees habitus, 10 x 10 m for pole habitus, 5 x 5 m for sapling habitus, and 2 x2 m for herbs and seedling habitus, and these samples were further divided into 4 plots at three habitats. These plots were distributed perpendicular to each hill gradient from swamp forest, mixed and primary forest. In order to analyze the habitat of three colored langurs, the established methods included vegetation analysis and the land coverage classification in accordance with the Ministry of Forestry and Environment (2021) that consisted of three main habitat types namely, secondary forest, swamp forest, and mixed forest. The vegetation analysis was further categorized into relative density (2), relative frequency (4), relative dominance (6), and the importance value index (INP) (4, 5) on each growth level of vegetation. Meanwhile, the availability of langur feed in the two ecosystems was determined based on the relative density value of each species. These values were utilized to establish the dominant species of a habitat (Parmadi et al. 2016).
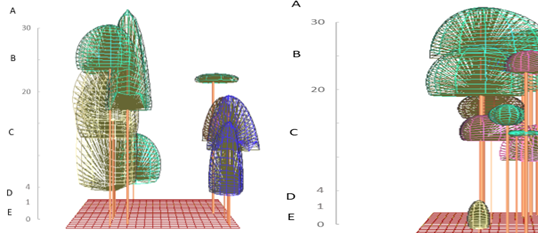
To illustrate the structure of habitat langurs the profile diagram by SeXiFS software (Hardja and Vincent 2008) was implemented. Data collected to create the structure profile using this software consist X: the x position of the tree base (m), Y: the y position of the tree base (m), Species: the species label, if the label is match with the one in the species list, then it will be linked, otherwise new species definition will be created, DBH: the diameter at breast height of the tree (m), Height: the height of the tree (m), CR Depth: crown depth (m), CR Curve: crown curve (m), CR Radius: crown radius in vertical projection, can be more than one value separated by semicolon (m), Rotation: a rotation of the vertical projection of the crown geometry (degree), CP: crown position index (0 - 1), CF: crown form index (0 - 1). This illustration will exhibit the vertical model of the ranging pattern by three colored langurs. The tree canopy stratum used by three colored langurs was further classified into several strata, namely stratum A (>30 m), stratum B (20 - 30 m), stratum C (4 - 20 m), stratum D (1 - 4 m), and stratum E (0 - 1 m) (Soerianegara and Indrawan 1988). Method to identify the daily and social activities was use scan sampling with 1 minute interval time to record each activity of an individual in a group at a certain time cumulatively (Hepworth & Hamilton 2001). To study the behavior pattern of the three colored langurs, continuous recording was practiced. Each behavior pattern was categorized into eating (take and eat foods to the mouth), moving (movements from one to another site using their quadrupedal), resting (off from all their activity and it can be indicated by the closed eyes or sleeping time), and social activity (Napier and Napier 1967). The social behavioral pattern was further classified into agonistic, vocalization, playing, exploring, and sexual. Each behavior was calculated as a percentage of its frequency and duration.
RESULTS AND DISCUSSION
Study Site and Time
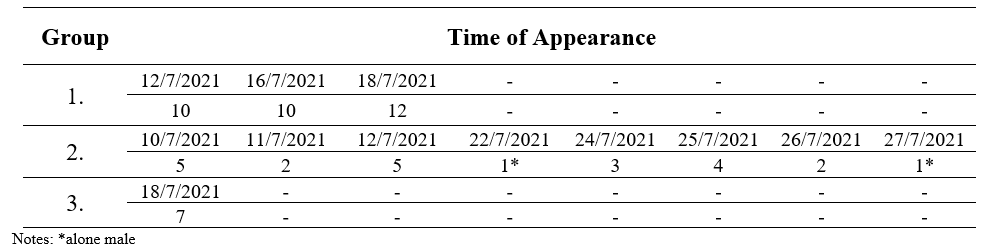
The field observation recorded three groups of langurs with total 16 encounters. Each group consisted of 21 - 24 individuals and was widespread in several parts of Bukit Semujan. The first group recorded nine individuals, including an infant and a baby in the south hill.eanwhile, on the west side (second group) of the hill five individuals were observed. Third group with seven individuals was recorded on the east of the hill, including one sole male with direct encounter who was observed more than two times always ranging alone at the surrounding camp research. This individual langur always observed around the group 2, so we notes the appearance at group 2. We have assumed that the alone male langur is a sub-adult individual and was rejected by the original group. It can be indicated and visible from body-size and genitals that are not too big and clearly visible. Following is the group composition of langurs inhabiting the study site during the preliminary study (Table 1).
Habitat Characteristic
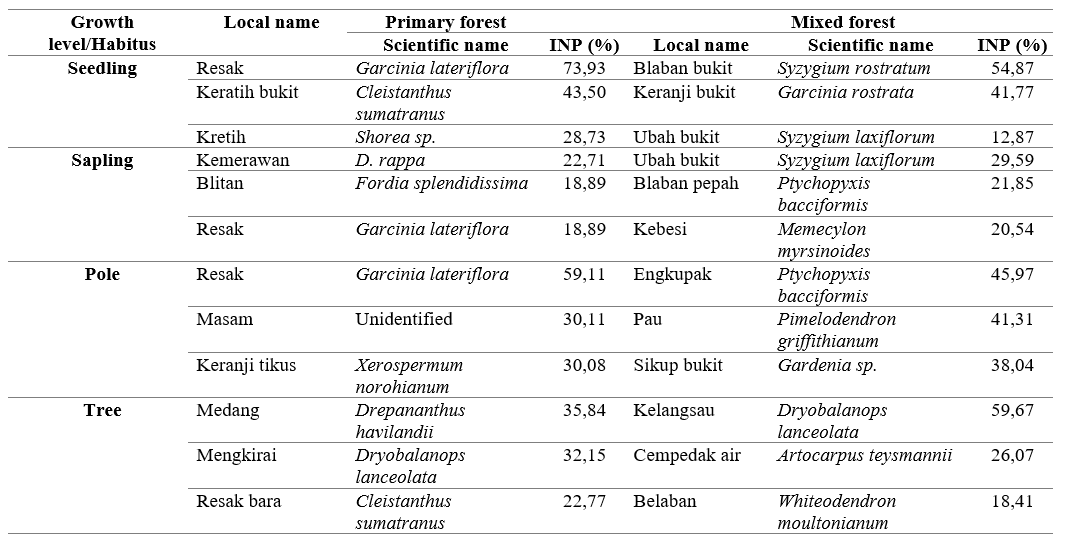
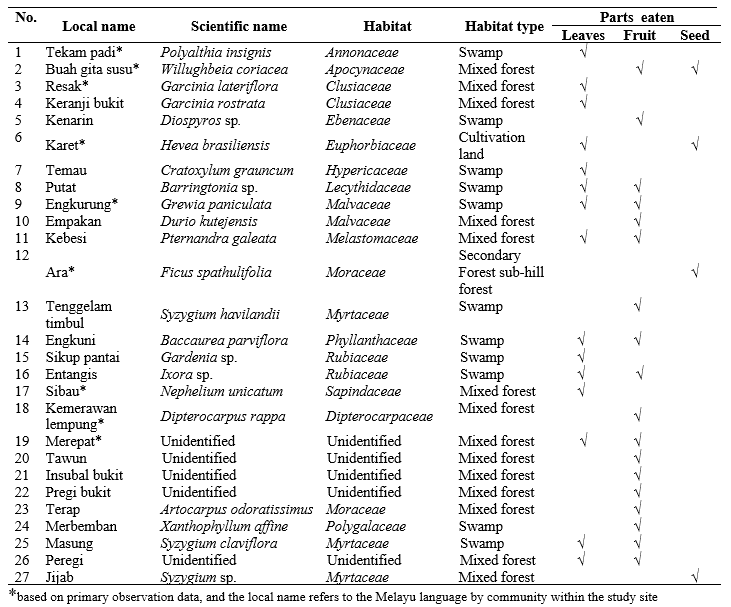
The habitat of the three colored langurs in Bukit Samujan, Lupak Mawang Resort has been distinguished in two distinct ecosystems, namely, the primary forest, and the mixed forest that occurs between swamp and sub-hill forest. Based on the results of vegetation identification in both ecosystems, there were 27 plant species that were found directly consumed as langur feeds. There are three species for each growth level that have the highest value index in both ecosystems, which signifies the cruciality of these species and its relevance in langur feeds for their future regeneration. The types of vegetation in the primary forest with the highest index value have been depicted in Table 2 below.
Habitat characteristics of primary forest is that it has thicker and firmer tree canopy than mixed forest. These characteristics not only play a significant role as a feed source, but also functions as a sleeping tree and aids in easier movement during their locomotion (Febriyanti 2008). Arboreal primates will choose sleeping trees based on the proportion and thickness of branches (Giovana 2015). Three colored langurs use the lush and tall canopy to acquire shelter from the sun during the day. The mixed forest has a good continuity canopy but is divided into several segments. It restricts langurs’ movements to lower planes and constraints them to rotate to reach other tree crowns.
Species and Feed Availability
Based on the results, there were 9 feed species that were recognized to be directly consumed by langurs in the study area during the observation, and the remaining 18 species were acquainted with the local community information. For the preliminary study, this is a big potential for diverse information and data about three colored langur’s feed species, especially during fruiting season. The following table contains the species list of langur’s feed and their respective fragments that were consumed during the preliminary study (Table 4).
The Myrtaceae family tree was found to be the chief feed resource among the tree species. Previous research found 19 species of feed trees in the same location. The dominant feed belonged to Clusiaceae, Moraceae, Anacardiaceae, and Euphorbiaceae (Musyaffa 2020). The types of feed highly preferred were Gita susu (Willughbeia coriacea), Merepat (unidentified), and Karet (Hevea brasiliensis). Based on these feed data, there is some unusual feed like H brasilliensis. H brasiliensis was present a long time before the study areas have become a National Park, and previous status as a nature and wild reserve from 1981–1983 . H brasiliensis was planted by the local community that has indigenous land surrounding the Bukit Semujan. Nevertheless, consumption of rubber seed during the preliminary study was staggering, however, field observations confirmed that langurs not only eat the seeds but also the young leaves of the rubber tree. However, langurs can only consume the already planted ones now due to the new regulations set by the National Park that restrict further planting of rubber trees in the protected land area.

The feed part for consumption included leaves, fruits, and seeds. Three colored langurs primarily consumed leaves (50%) in comparison to other parts, such as fruits (30%), and seeds (20%). The genus Prebytis is one of the primates that eat fruits and leaves, however mainly prefers leaves parts (Sumarni 2016). Presbytis chrysomelas ssp cruciger is the same as their relative species in one genus, namely P. comata and P. hosei (Ruhiyat 1983; Mitchell 1994). The type of feed consumed by three colored langurs was dominant from tree species (89%) and lianas (11%). Three colored langurs chiefly consumed leaf of rice tekam (Polyalthia insignis). Some leaf species have a complete source of nutrients including protein, carbohydrates, fat, tannins, and water (Zulfahri and Pohan 2016). The species that consumed the fruit were Gita susu (Willughbeia coriacea), Sibau (Nephelium unicatum), and Karet (Hevea brasiliensis). Three colored langurs usually consume fruits that are small to adequately sized with range diameter 0,5 – 5 centimeter and lightly colored. Primates did like fruits with hard skin, cracked, and yellow to brown color (Leighton & Leighton 1983). In addition to the leaves and fruits, three colored langurs also consume the seeds of several types of feed species. Seeds of Karet (Hevea brasiliensis) are a rich source of forage for langurs. The water, protein, fat, and crude fiber content is beneficial for the optimum metabolism required for the growth of langur (Syamsunarno & Sunarno 2019).
The potential of feed availability in both habitats can be determined through the relative density of each species. It was influenced by physical and biotic habitat factors and disturbances from destructive activities (Violita et al. 2015). The relative density of a plant species will determine the dominance of that species in a community (Putri & Sudrajat 2017). The results of the relative density of forage plant species in the primary forest are shown in Table 5.
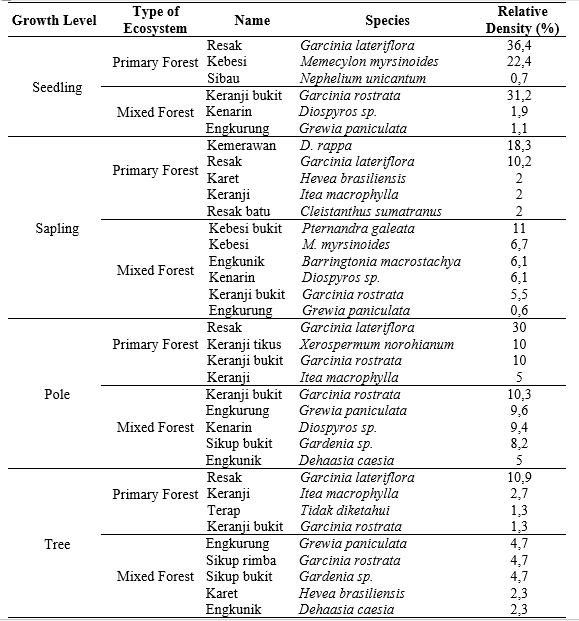
The relative density of feed plants in the primary forest was 9 species of seedlings, 20 species of saplings, 11 species of poles, and 23 species of trees. The seedling and sapling density could be an indicator of feed availability in the future, while the pole and tree level indicate the current availability of feed (Shankar 2001). The feed species with high regeneration capacity in a primary forest is Resak (Garcinia lateriflora). This species has a high relative density at every growth level. On the other hand, Keranji (Itea macrophylla) has an overall high relative density at all growth stages, except the seedling level.
Meanwhile, the relative density of mixed forest was 16 species of seedlings, 16 species of saplings, 13 species of poles, and 18 species of trees. The feed species with high regeneration capacity is Engkurung (Grewia paniculata). The other species with good regeneration and inadequate growth rates were Keranji bukit (Garcinia rostrata) and Kenarin (Diospyros sp.). Then, the species that did not have good regeneration were Sikup rimba (Garcinia rostrata) and Karet (Hevea brasiliensis). The species like G panculata, G rostrata and Diospyros sp with high regeneration need to increase, and species with low regeneration either.
Daily Activity
Lutung sentarum begins activity at dawn (06.00) until early evening (18.00), adding the total time for their activity to 320 minutes. This diurnal primate will give signal communication to a movement for their group, called a morning call. This behavior exhibits in other species too such as P. thomasi, which emits a morning call from a sleeping tree (Wich et al. 2002). During midday between 12.00–13.00, the three colored langurs will tend to find a place for rest after foraging in the morning. Foraging in the morning would increase body temperature with air temperature (Prayogo 2006). Their activities were divided into foraging, moving, resting, and social. The percentage of each activity is divided into three parts of times, namely morning (06.00–10.00), afternoon (10.01–14.00), and afternoon (14.01–18.00) in Figure 3.
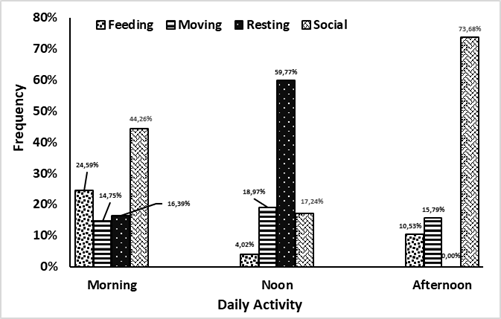
Three colored langurs allocate more time to do certain activities, especially social behavior and foraging in the morning. Some activities usually coincide with another activity. In addition, the langurs will keep moving until they find suitable feed trees that the group needs (Figure 4a). The movement gesture of langurs was rarely quadrupedal, walking using all four legs and arms. This movement could be down and up or to move to other trees by jumping. An adult male as the group leader always led the group movement. However, in some cases, it has been found that adult females with babies would lead the group movement. In all circumstances, juvenile or young individuals are restricted from leading the group (Nursal 2001).
Lutung sentarum will rest more during the day after foraging (Figure 4b). This can be attributed to several factors, ranging from the process in the body’s metabolism after eating to the influence of air temperature that affects body condition (Alikodra 1990; Prayogo 2006). Lutung sentarum spent 1–2 hours resting and sleeping during the day. The rest location usually chosen had a sturdy branch or a dense canopy, such as Mengkirai (Dryobalanops lanceolata) species. The resting position of the Lutung sentarum includes hunched sitting with one hand holding the trunk or branch of a nearby tree. It is also found in other primates, namely Trachypithecus auratus. The positions found were bending over with the head tucked into the stomach between the two knees of the legs, the soles of the feet overlapping each other and the hands holding the branches (Giovana 2015).

The activity of the Lutung sentarum in the afternoon dominates by social behavior, namely a collection of behaviors carried out by two or more individuals and interconnected to survive. This activity could be carried out with other species. These activities are divided into agonistic and affiliative (Sajuthi et al. 2016). Agonistic is a form of negative response to something like fights and coalitions. As for affiliation, it is a form of positive response such as grooming, playing, vocalization, and sexual activity. Based on the analysis, the social activity amount to 66 times with a total duration of 72 minutes. Agonistic is the most active behavioral pattern (48.48%) of social activities. This behavior is a gesture of response to becoming steady and grinning position. The group leader (alpha male) would exhibit this position with vocalizations. This posture aims to display objects that make langur feel threatened and to repel. Usually, this position can be sitting or bending the body toward other species to make the langur look big. Chimpanzees (Pan troglophytes) also exhibited it by straightening their body hair to make their posture look bigger and more dangerous (van Hooff 1973).
Vocalization is an expression by making sounds for other interspecies and environments (Irawan 2011). The vocals of three colored Lutung sentarum were identified into two categories, its sounds with loud and long characteristics aimed at disturbance as a form of threat. Then the voice with a shorter duration is addressed to group members as a form of disturbance warning. Both types of vocalizations belong to loud calls, vocalizations that are loud and prominent in the primate vocal repertoire (Delgado 2006). In addition, there are many functions of vocalization, namely as a marker of social status, efforts to maintain food sources, maintaining territory between groups, and maintaining cohesion between group members (Wich et al. 2002, Wich et al. 2003, Wich &Nunn 2022, Chiarello 1995, Riley 2005).
The playing activities most carried out by young males, included chasing and wrestling each other. Playing will train the motor nerves to avoid predators, protect themselves, and attract partners (Spinka et al. 2001). Then for grooming, it is usually done by adult female individuals to their babies. This activity shows proximity to each individual in groups. It is done by treating and searching for lice on the ears, neck, shoulders, and back with the hands, feet, teeth, and tongue (Napier & Napier 1985). Grooming is also good for health maintenance (Zamma 2002). Activities that were not found in this research were sexual. The fundamental of living things was to obtain and maintain populations of their species according to the maturity level of an individual. Lutung sentarum are polygamous and practices polygynous mating system, so mating does not refer to the mating season like other species, namely Macaca fascicularis (Setiawan 2002).
Stratum Use on Daily Activity
Based on the encounters with three colored langurs, their distribution comprehends several areas of primary and mixed forest. This movement has been influenced by factors of feed, shelter, and a safe place for langur groups. During afternoons, three colored langurs tend to move to the hills because of the tall trees that are more than 30 meters in height with lush canopy. Using the stratum of three colored langurs in the primary and mixed forest had different purposes based on their activities. Trees for three colored langurs as arboreal animals are important, especially for rest, self-protection, foraging, mating, and socializing. The results showed that most of the canopy proportions used by three colored langurs were stratum C (70.49%) and stratum B (27.87%). It was similar to the previous study in which three colored langurs used stratum C (78%) dominantly (Musyaffa 2020). The stratum utility is presented in Figure 5.
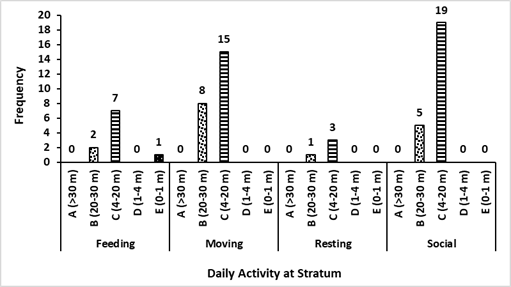
Stratum C was a place for foraging activity that included seven encounters with the langur group there. The longest resting time is about 103 minutes. Mengkirai (Dryobalanops lanceolata) is one of the sleeping trees, and the langur group was found at an altitude of 30 meters on the Mengkirai tree. In addition, there was a case in this study that Lutung sentarum would descend to the stratum E (ground) to pick up fallen food. The moving activity of three colored langurs has been carried out in stratum B and C for supply needs that avoided disturbance. The canopy has a function to make it easier for the Lutung sentarum to move through connected tree branches. Other primates, such as the Javan gibbon, use a dense canopy to support their cruising range (Zanuansyah 2013).
In addition, the social activities mostly had a similar stratum to other activities. The langur appearance on stratum C and B amounted to 19 times and five times during the research respectively. Vocalization was the most common activity; the sounds were louder and echoed further. Other species, such as the Trachypithecus auratus will choose trees with upper-middle crowns for vocalization so that the sound echoes further and clearer (Oktaviani 2009). The use of ranging patterns by three colored langurs in primary and mixed forest habitats is exhibited in Figure 6. In addition, this habitat is inhabited by other species, especially in the primate class. According to ecological theory, the concepts of niche and resource competition are central to the coexistence of sympatric species (Gause 1934; Tokeshi 1999).
Conservation Implication
For conservational efforts, feeding ecology is crucial including the type and composition of feed in its natural habitat not only for better understanding but also to conduct habitat enrichment, security and further research related to bio-ecology and conservation of langur in the natural habitat by the long-term National Park managers and interested parties. As we are aware, primates do not receive the required research attention and are often neglected due to arduous and restricted collection of primary data in their habitat. This preliminary study is a progressive step forward toward future research and to draw the attention of researchers and observers to conduct numerous studies on primate species that are currently lacking the bio-ecology data.
CONCLUSION
The habitat of the three colored langurs in Bukit Semujan, Lupak Mawang Resort was discovered in both primary as well as mixed forests. The feed species for three colored langurs in both habitats amounted to 27 species, and the highly preferred are Gita susu (Willughbeia coriacea), Merepat (unidentified), and Karet (Hevea brasiliensis). Its feed existed in study areas because of prior sowing by the local community before the land was distinguished as part of the National Park. Meanwhile, the highly preferred feed compositions were leaves (50%), fruits (30%), and seeds (20%). The highly preferred stratum for their activities was stratum C (70.49%) and B (27.87%). The identification of vegetation species for resting or sleeping was Mengkirai (Dryobalanops lanceolata). The highest daily activity of three colored langurs is divided into three parts of time, the morning dominated by social activity (44.26%), the day dominated by resting (59.77%), and the afternoon dominated by social activity (73.68%). The social activity most frequently exhibited by Lutung sentarum was agonistic (48.48%), vocalization (39.39%), playing (10.61%), searching (1.52%), and sexual (0 %).
REFERENCES
- Alikodra HS. 1990. Pengelolaan Satwa Liar Jilid 1. Bogor: Institut Pertanian Bogor.
- Chiarello AG. 1995. Role of loud calls in brown howlers, Alouatta fusca. American Journal of Primatology 36(3): 213–222. DOI: 10.1002/ajp.1350360305
- Delgado RA. 2006. Sexual Selection in the Loud Calls of Male Primates: Signal Content and Function. International Journal of Primatology. 27(1): 5–25
- Febriyanti NS. 2008. Cover Characteristic study of Ebony Leaf Monkey (Trachyphithecus auratus Geoffroy 1812) at Blok Ireng-Ireng, Bromo Tengger Semeru National Park, East Java [undergraduate thesis]. Bogor: IPB University.
- Gause GF. 1934. The struggle for existence. New York: Hafner
- Giovana D. 2015. Daily Behavior and Homerange of Ebony leaf monkey (T. auratus Raffles 1821) in Bama Resort Baluran National Park [Undergraduate Thesis]. Bogor: IPB University.
- Hadi S, Ziegler T, Waltert M, Syamsuri F, Mühlenberg M, Hodger JK. 2012. Habitat Use and Trophic Niche Overlap of Two Sympatric Colobines, Presbytis potenziani and Simias concolor, on Siberut Island, Indonesia. International Journal Primatology. 33(1): 218–232. DOI: 10.1007/s10764-011-9567-y
- Harja D and Vincént G. 2008. Spatially Explicit Individual-based Forest Simulator-User Guide and Software. World Agroforestry Centre (ICRAF) and Institut de Recherche pour le Développement (IRD).
- Hepworth G, Hamilton AJ. 2001. Scan sampling and waterfowl activity budget studies: design and analysis considerations. Behavior. 138: 1391–1405. DOI: 10.1163/156853901317367654
- Houle A, Vickery WL, Chapman CA. 2006. Testing mechanisms of coexistence among two species of frugivorous primates. Journal of Animal Ecology. 75(4): 1034–1044. DOI:10.1111/j.13652656. 2006.01125.x
- van Hooff, J. 1973. A Structural Analysis of The Social Behavior of A Semicaptive Group of Chimpanzees. London: Academic Press
- Irawan A. 2011. Daily Behaviour Activity of Male Maroon Leaf Monkey (Presbytis rubicunda) During The Day in Captivity [Undergraduate Thesis]. Bogor: IPB University.
- Leighton M, Leighton DR. 1983. Vertebrate Responses to Fruiting Seasonality within a Bornean Rain Forest. Tropical Rain Forest: Ecologycal and Management. 39(4): 485–503.
- Ministry of Forestry and Environmental [KLHK]. 2014. Zonation of Danau Sentarum National Park. Putussibau.
- Mitchell AH. 1994. Ecology of Hose’s langur, Presbytis hosei, in mixed logged and unlogged dipterocarp forest of north Borneo [tesis]. New Haven (US): Yale University.
- Musyaffa MEF, Santoso N. 2020. Habitat Characteristics and Activity Patterns of Cross-marked Langur (Presbytis chrysomelas cruciger) in Danau Sentarum Nasional Park. Journal Penelitian Hutan dan Konservasi Alam. 17(2): 155–172. DOI: 10.20886/jphka.2020.17.2.155–172
- Napier JR, Napier, PH. 1967. A handbook of living primates: morphology, ecology and behaviour of nonhuman primates. London: Academic Press
- Napier JR, Napier PH. 1985. The Natural History of the Primates. Massachusetts: MIT Press.
- Nijman V, Cheyne S, Traeholt C, Setiawan A. 2020. Presbytis chrysomelas ssp. chrysomelas. The IUCN Red List of Threatened Species 2020: e.T136857A17987458. DOI: 10.2305/IUCN.UK.2020-2.RLTS.T136857A17987458.en
- Nursal WI. 2001. Daily activity of Javan langur (Trachypithecus auratus, Geoffroy 1812) at Selabintana Pos in Gunung Gede Pangrango National Park, West Java [Undergraduate Thesis]. Bogor: IPB University
- Oktaviani R. 2009. Study of Calling Behavior in Javan Gibbon (Hylobates moloch Audebert, 1798) at Gunung Halimun Salak National Park, Province of West Java [Undergraduate Thesis]. Bogor: IPB University.
- Parmadi EH, Dewiyanti I, Karina S. 2016. Important Index Values of Mangrove in Kuala Idi, East Aceh Regency. Jurnal Ilmiah Mahasiswa Kelautan dan Perikanan Unsyiah. 1(1): 82–95
- Zulfahri MR, Pohan SD. 2016. Analysis of Orangutan feed (Pongo abeli) at Resort Sei Betung Sumatra Utara, Gunung Leuser National Park. Jurnal Biosains. 2(2): 97–103. DOI: 10.24114/jbio.v2i2.4222
- Prayogo H. 2006. Study on behavior and food analysis of silver leaf monkey (Trachypithecus cristatus) at Schmutzer Primate Center, Ragunan Zoological Gardent [Magister Thesis]. Bogor: IPB University
- Putri KP, Sudrajat DJ. 2017. Regeneration of Shorea spp. in the Seed Sources of KHDTK Haurbentes. Bogor District. Jurnal Perbenihan Tanaman Hutan. 5(2): 71–79. DOI: 10.20886/bptpth.2017.5.2.71-79
- Rifqi MA, Pambudi T, Khotiem M, Gesshaa AA. 2019. Bornean banded langur Presbytis chrysomelas cruciger in Danau Sentarum National Park, West Kalimantan, Indonesia. Southeast Asia Vertebrate Records. 56–59
- Riley EP. 2005. The Loud Call of the Sulawesi Tonkean Macaque, Macaca tonkeana. Tropical Biodiversity. 8(3): 199–209.
- Roslinda E. 2013. Management policy options of Danau Sentarum National Park West Kalimantan Province [Magister Thesis]. Bogor: IPB University.
- Ruhiyat Y. 1983. Socio-ecological study of Presbytis aygula in west Java. Primates. 24(3): 344–359. https://psycnet.apa.org/doi/10.1007/BF02381980
- Sajuthi D, Astuti DA, Perwitasari D, Iskandar E, Sulistiawati E, Suparto IH, Kyes RC. 2016. Primate Display Model: Macaca fascicularis (Study on Population, Behaviour, Status Nutrient, and Nutrient for Deseases Model). Bogor: IPB Press http://repository.ipb.ac.id/handle/123456789/81590
- Schoener TW. 1974. Resource partitioning in ecological communities. Science. 185(4145): 27–39. DOI: 10.1126/science.185.4145.27
- Setiawan I. 2002. Sexual behavior of long-tailed macaque (Macaca fascicularis) on makam keramat Solear, Tangerang. [undergraduate thesis]. Bogor: IPB University.
- Shankar U. 2001. A case of high tree diversity in Sal (Shorea robusta) dominated lowland forest of eastern Himalaya: Floristic composition, regeneration dan conservation. Current science. 81(7): 776–786.
- Singh M, Royi K, Singg M. 2011. Resource partitioning in sympatric langurs and macaques in tropical rainforests of the central Western Ghats, South India. American Journal of Primatology. 73(4): 335–346. DOI: 10.1002/ajp.20900
- Soerianegara I, Indrawan A. 1988. Ekologi Hutan Indonesia. Bogor: Departemen Manajemen Hutan Fakultas Kehutanan IPB
- Spinka M, Newberry RC, Bekoff M. 2001. Mammalian play: training for the unexpected. The Quarterly Review of Biology. 76(2):141–68. DOI: 10.1086/393866
- Sumarni S. 2016. Study on feed species of kelasi (Presbytis rubicunda) at forest tourism areas in Baning, Sintang Regency. Piper. 23(12): 115–124. DOI: 10.51826/piper.v12i23.25
- Syamsunarno MB, Sunarno MTD. 2019. Study on Rubber Seed Hevea brasiliensis as A Candidat of Fish Feed Ingradient. Jurnal Ilmu Pertanian dan Perikanan. 3(2): 135–142. http://umbidharma.org/jipp
- Tokeshi M. 1999. Species coexistence: Ecological and evolutionary perspectives. Oxford: Blackwell Science.
- Violita CY, Setiawan A, Rustiati EL. 2015. Simpai (Presbytis melalophos) group size in a forest of cugung village of protection forest management unit model of rajabasa mountain south lampung. Jurnal Sylva Lestari. 3(3): 11–18. DOI: 10.23960/jsl3311-18
- Watanabe K. 1981. Variations in group composition and population density of the two sympatric Mentawaian leaf-monkeys. Primates. 22:145–160. DOI: 10.1007/BF02382606
- Wich SA, Assink PR, Becher F, Sterck EHM. 2002. Playbacks of loud calls to wild Thomas langurs (Presbytis thomasi): the effect of familiarity. Behaviour. 139(1): 79–87. DOI: 10.1163/15685390252902292
- Wich SA, Nunn CL. 2002. Do male “long-distance calls” function in mate defense? A comparative study of long-distance calls in primates. Behavioral Ecology and Sociobiology. 52(6): 474–484. DOI: 10.1007/s00265-002-0541-8
- Wich SA, Koski S, de Vries H, van Schaik CP. 2003b. Individual and Contextual Variation in Thomas Langur Male Loud Calls. Ethology. 109(1): 1–13. DOI: 10.1046/j.1439-0310.2003.00837.x
- Zamma K. 2002. Grooming site preferences determined by lice infection among Japanese macaques in Arashiyama. Primates. 43(1):41–49. DOI: 10.1007/bf02629575
- Zanuansyah A. 2013. Locomotion Behavior and Posture of Javan Gibbon (Hylobates moloch Audebert, 1798) in Gunung Halimun Salak National Park [Undergraduate Thesis]. Bogor: IPB University.