INTRODUCTION
Plants provide an incredible source of new medicinal product inventions for development. One of the medicinal plants often used by the community is Java ginseng (Talinum paniculatum Gaertn.) from the Portulacaceae family. Java ginseng is widely used as a substitute for Korean ginseng, which continues to be imported, because it is relatively cheap, easy to obtain, and easy to cultivate (Widiyani, 2006). The plant’s chemical content comprises saponins, triterpenes, polyphenols, and essential oils (Komatsu, 1982). The root of the Java ginseng plant is the part that can be used as a medicinal ingredient. The most important and dominant component in the chemical content of Java ginseng root is saponins. Talinum paniculatum Gaertn. has very slow root growth in its natural habitat, requiring around two to three years to produce 100 g of roots per plant (Manuhara et al., 2015). Therefore, in vitro culture techniques offer potential as an alternative means of accelerating the root growth of this plant.
Root growth can be increased by manipulation in culture media with the addition of nutrients (Manuhara, 2014). As such, in this study, an increase in root biomass was achieved with the addition of phosphate and nitrogen sources. Phosphate has an important function in plant growth due to its role in transferring energy molecules such as ADP and ATP, NAD and NADP, as well as genetic information system compounds such as DNA and RNA (Barker and Pilbeam, 2007). In plant tissue culture, the concentration of phosphate in the medium can be a major factor influencing plant growth. According to Dormatey et al. (2021), phosphite supply in the Murashige and Skoog (MS) medium influenced root morphological characteristics and fresh biomass in five genotypes of potatoes. Furthermore, Pavlov et al. (2000) stated that Lavandula vera biomass and rosmarinic acid was maximally produced with the addition of a two-time phosphate concentration in MS media.
Apart from phosphate, nitrogen sources also affect cell growth and the formation of secondary metabolites. Nitrogen functions as a component of amino acids, proteins, and nucleic acids in plants (Wiedenhoeft, 2006). Ammonium (NH4+) and nitrate (NO3-) are used as the main sources of nutrition in plant cell and tissue culture (Zhang et al., 1996, in Kim et al., 2005). When both nitrogen sources were administered simultaneously, growth and yield were significantly increased compared to ammonium or nitrate alone (Zhang et al., 2011). Research by Yin et al. (2013) on Pseudostellaria heterophylla plants showed that the highest adventitious root biomass, namely 9.11 g fresh weight and 0.54 g dry weight, was obtained with an ammonium and nitrate ratio of 20:40. Furthermore, Panda et al. (1992) reported that the only received information on ammonium was used as not only the only nitrogen source. Therefore, it is very important to determine the optimal ratio of ammonium to nitrate. No study has reported the effect of various concentrations of phosphate and the ammonium:nitrate ratio on the biomass production of Javanese ginseng plants. This study therefore aims to investigate their effect in culture media on the adventitious root biomass of Javanese ginseng.
MATERIALS AND METHODS
Adventitious root induction from leaf and stem explants
The adventitious roots of Java ginseng were induced from stem and leaf explants grown in solid MS medium supplemented with 30 g/L sucrose, 6 g/L agar, and various combinations of IBA growth regulator 2 mg/L with three types of cytokinins, namely BAP (0.1, 0.3, and 0.5 mg/L), kinetin (0.1, 0.3, and 0.5 mg/L), and TDZ (0.1, 0.3, and 0.5 mg/L). The leaf samples (1 cm2) were taken from the second and third leaves of the shoots from intact plant. All explants were immersed in detergent solution for 3 minutes and rinsed with water. The explant surfaces were sterilized by immersing them in 10% Clorox solution for 10 minutes. The explants were then washed 3 times using sterile distilled water. Following this, they were maintained in an incubation room with an average temperature of 25oC in dark conditions. After 28 days, the adventitious roots were harvested and the fresh weight, dry weight, number of roots, and duration of adventitious root formation were measured.
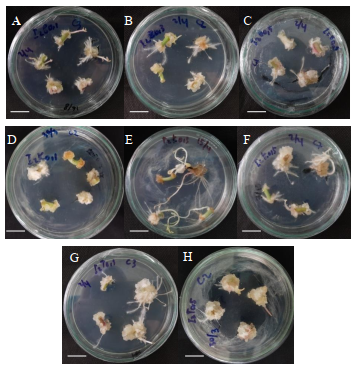
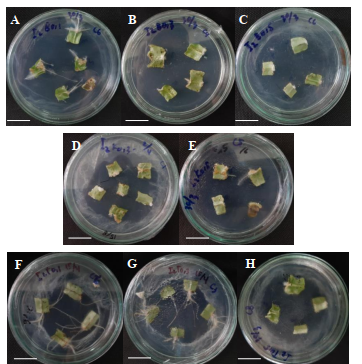
The stem and leaf explants induced from various combinations of growth regulator IBA 2 mg/L with the different types of cytokinins (BAP, kinetin, and TDZ) at separate concentrations (0.1, 0.3, and 0.5 mg / L) produced varied responses, as seen in Tables 1 and 2.
The fastest mean root formation time was obtained from the induction of stem explants with the addition of a combination of growth regulator IBA 2 mg/L + kinetin 0.1 mg/L, namely in 4.67 days. Meanwhile, for the leaf explants, the fastest root formation time, at 11.67 days, was obtained by adding the combinations of growth regulator IBA 2 mg/L + kinetin 0.3 mg / L and IBA 2 mg / L + BAP 0.5 mg/L. Whereas the addition of a combination of growth regulator IBA 2 mg/L + TDZ 0.5 mg/L for stem explants and IBA 2 mg/L + TDZ 0.1 mg/L for leaf explants resulted in the longest mean times to root formation, namely 21 and 21.33 days, respectively.
Based on Table 1, the highest average fresh weight, dry weight, and number of roots for the stem explants were obtained in the IBA treatment of 2 mg/L + kinetin 0.3 mg/L. While the highest average root length of stem explants, namely 13.33 cm, was obtained in the IBA treatment of 2 mg/L + 0.3 mg/L TDZ. In Table 2, the greatest average root fresh weight was obtained from the results of leaf explant induction with the addition of a combination of growth regulator IBA 2 mg/L + TDZ 0.1 mg/L, namely 4.53 mg. Meanwhile, the highest dry weight, number of roots, and root length were obtained from the induction of leaf explants with the addition of a combination of growth regulator IBA 2 mg/L + TDZ 0.1 mg/L. The lowest average dry weight was obtained with the combination of IBA 2 mg/L + BAP 0.3 mg/L, namely 0.1 mg.
Adventitious roots can be induced from various explants including leaves, stems, roots, and various other factors such as auxins (Baque et al., 2010). The selection of the types, concentrations, and combinations of growth regulators is very important. Based on previous research, adventitious roots have been successfully induced from Javan ginseng leaf explants on a solid MS medium with the addition of IBA 2 mg/L, which produced the highest root mass of 5.929 g (Solim et al., 2017 and Manuhara, 2016). Meanwhile, Erin et al. (2020) reported that the treatment of IBA 2 mg/L + ethephon 1 mg/L produced the highest average number of roots, namely 7.33, compared to other treatments.
BAP and kinetin hormones are chemical compounds that are included in the cytokinin group and play a role in shoot growth. This time, however, they were combined with auxin IBA to stimulate adventitious root growth on T. paniculatum stem and leaf explants. In the research of Isda and Fatonah (2014), the highest number of roots was found at a BAP concentration of 0.5 mg/L + 1.0 mg/L NAA, namely 5.00 fruit on the explants of Grammatophyllum scriptum orchid shoots. However, in the results of this study on stem and leaf explants, the combination of IBA 2 mg/L + BAP 0.5 mg/L did not provide optimal results in all parameters of the observation. It is thus evident that the effect of these growth regulators depends on the type of plant and the dosage concentration of ZPT growth regulator combination that is suitable; as such, for T. paniculatum this is not the optimal concentration of auxin and cytokinin combination to produce the most roots. Apart from BAP, kinetin is also often combined with the auxin hormone in its use in vitro, as in the research of Mahadi et al. (2013) where the highest average number of dragon fruit explant roots was found in the N0.4 K4 treatment, namely 5.25 roots. However, the lowest average number of roots was also found in the N0.4 K4 treatment. This is presumably because high kinetin administration can produce stunted explant growth; Wahidah (2011) stated that kinetin hormone can affect the process of plant development at low concentrations while inhibiting growth at high concentrations.
TDZ can play a role in stimulating endogenous cytokinin production. Therefore, TDZ can increase the action of other cytokinins, both exogenous and endogenous cytokinins (Guol et al., 2011). The administration of TDZ at a low concentration induces callus faster than at a high concentration; for callus regeneration, it is better to combine TDZ and NAA at low concentrations than TDZ alone (Oláh et al., 2003). This is corroborated by the findings of this study, where TDZ with a concentration of 0.1 mg/L induced the formation of a large number of adventitious roots to produce a large fresh weight and dry weight compared to concentrations of 0.3 mg/L and 0.5 mg/L. The formation of adventitious roots is a type of positive synergy between TDZ and IBA as the best adventitious root-forming hormone.
Effect of phosphate concentration (KH2PO4) on adventitious root growth
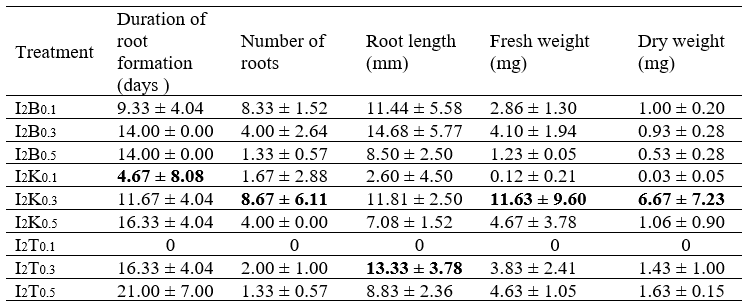
The best treatment for inducing root growth (a combination of IBA 2 mg/L and TDZ 0.1 mg/L) was used to determine the effect of phosphate concentration and the ammonium:nitrate ratio on adventitious root growth. The average fresh weight, dry weight, root growth, number of roots, and adventitious root length of T. paniculatum in various phosphate concentration treatments of MS medium are listed in Table 3.
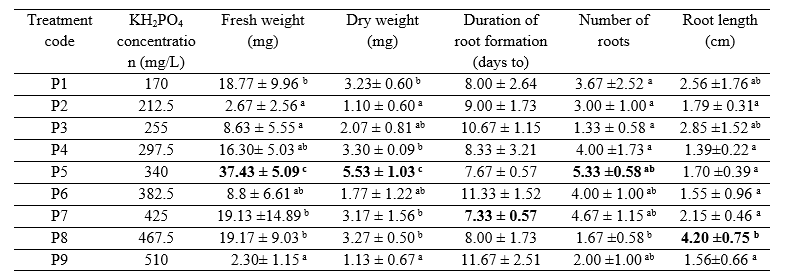
The highest average values for fresh weight, dry weight, and the number of roots were obtained for the P5 treatment (phosphate concentration 340 mg/L). Meanwhile, the fastest root growth rate of 7.3 days was achieved with the P7 treatment (phosphate concentration 425 mg/L), and the longest average root is shown for the P8 treatment (phosphate concentration 467.5 mg/L). The highest average fresh weight and dry weight values were identified for the P5 treatment. This is consistent with research conducted by Curtis et al. (1991), which stated that the growth of Opium poppy in cell suspension culture increased by 50% in media with two times the concentration of phosphate added. This occurred since phosphate plays an essential role in the transfer of energy for cell metabolism, the constituents of cell membranes, and nucleic acids. The lowest fresh weight was found on the media with the P9 treatment (510 mg/L) due to the very high phosphate concentration. The plant cells in these explants were stressed due to the very high salt concentration. The increase in the plant’s dry weight is attributable to the nutrients that were absorbed by the root and the accretion of protoplasm due to the increase and size of the cell count (Khristyana et al., 2005).
The fastest average duration of root formation was 7.3 days (phosphate concentration 425 mg/L) based on the application of the control treatment (phosphate concentration 425 mg/L) for 8 days. In this study, the P9 treatment (phosphate concentration 510 mg/L) showed the longest average duration of root formation (11.6 days) because media with high phosphate concentrations can suppress growth (George et al, 2007). Phosphate can bind with calcium and other microelements to reduce the absorption of other elements below the maximum level (Buddh, 2014).

The number of roots produced in each explant is different (Figure 3). The highest mean number of roots was found in the explants treated with P5 (KH2PO4 340 mg/L). The treatment in this study was controlled by P1 (170 mg/L of KH2PO4). The number of roots stood at only 2.56; thus, while both were from the second and third leaves of the shoots, they were from different plants. Auxin plays a role in cell elongation, cell division, and adventitious root formation (George, 2007). Therefore, different endogenous auxins lead to differences in root formation, the number of adventitious roots, and the length of adventitious roots.
Effect of ammonium:nitrate ratio on adventitious root growth
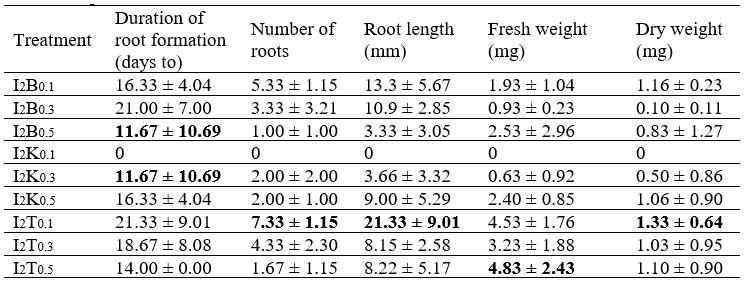
The average fresh weight, dry weight, root growth, number of roots, and the adventitious root length of T. paniculatum in various ammonium:nitrate ratios on MS medium are listed in Table 4.
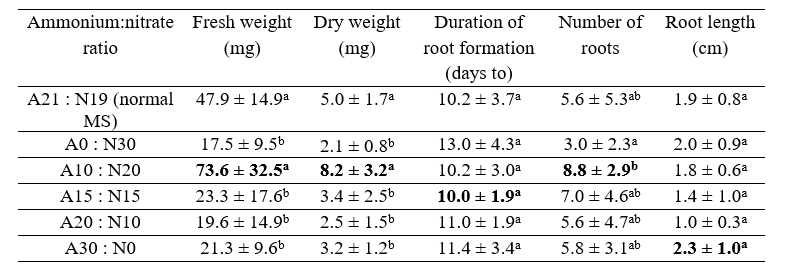
The highest average fresh weight, dry weight, and number of roots were obtained for the ammonium:nitrate ratio of 10:20. Meanwhile, the fastest average time to root formation was 10 days and the longest root length was 2.3 cm. However, the data showed no significant difference between all treatments, meaning the data did not affect the adventitious root biomass of Java ginseng.
Figure 4 contains pictures of 6 different ammonium:nitrate ratios, namely 21:19 mM as the control, and 0:30 mM, 10:20 mM, 15:15 mM, 20:10 mM, and 30: 0 mM for the adventitious roots of Java ginseng at 6 weeks.

Adventitious roots (i.e., roots that form from non-root tissue) can arise in various tissue locations from groups of mature cells that renew their cell division activity (Taiz and Zeiger, 2003). In the treatment ratio ammonium:nitrate 10:20, NH4Cl has good potential to replace NH4NO3 in control as a nitrogen source to increase adventitious root biomass production. This is because nitrogen is used for protein synthesis, both structural and enzymatic, and is thus needed for cell and organ growth, including for the production of plant biomass (Lawlor et al., 2001). In addition, the form and amount of nitrogen in the in vitro media have a significant effect on the rate of cell growth, differentiation, and cell totipotency (Kirkby and Mengel, 1987).
The average time to root formation in this study ranged from 10 to 13 days (Table 4). This aligns with the findings of research by Palestine (2008) on pule pandak (Raufolvia serpentine, L.) showing that the addition of IBA with a concentration of 2 to 4 mg / L can initiate root growth faster than other treatments, namely in 15 days.
The highest number of roots was found in the ammonium:nitrate ratio of 10:20, while the lowest number of roots was found in the ammonium:nitrate ratio of 0:30. Efforts to increase the number of roots include the addition of auxin growth regulators that can stimulate root induction. Wattimena (1988) explained that auxin is a plant hormone essential for cell division and root formation. Root emergence is influenced by the number of roots and correlates with the absorption of nutrients present in the culture medium.
Several studies have shown that nitrogen compounds and the ratio between ammonium and nitrate can affect the differentiation, de-differentiation, growth, and development of explants, as well as organ formation (Preece, 1995). The average root lengths of the treatments in this study are shown in Table 4; they range from 1 cm to 3 cm with no influence between one treatment and another. The increase in plant size reflects the increase in protoplasm that occurs due to the increase in cell size and number (Khristyana et al., 2005).
CONCLUSION
The combination of IBA 2 mg / L + kinetin 0.3 mg / L is the optimal concentration to produce the highest mean number of roots, fresh weight, and dry weight of Talinum paniculatum adventitious roots in stem explants. Meanwhile, for leaf explants, the best results were obtained with the adding combination of IBA 2 mg / L + Thidiazuron 0.1 mg / L. The highest fresh weight (37.47 mg) and dry weight (5.53 mg) were obtained in the phosphate concentration (KH2PO4) 340 mg/L treatment. Meanwhile, the ammonium:nitrate ratio of 10:20 was the best treatment to produce the highest biomass (fresh weight 73.6 mg and dry weight 8.2 mg).
ACKNOWLEDGMENTS
This research was funded by the Directorate General of Higher Education, Research and Technology No. 852/UN3.14/PT/2020 in the Master’s Thesis Research scheme.
REFERENCES
- Baque MA, Hahn EJ, Paek KY. 2010. Induction of adventitious root from leaf explants of Morinda citrifolia affected by auxin and light quality. In Vitro Cell Dev Biol Plant 46:71–80.
- Barker AV, Pilbeam DJ. 2007. Handbook of plant nutrition. Boca Raton: CRC Press, Taylor & Francis. p. 52.
- Buddh S. 2014. Comparative study of rock phosphate and calcium phosphate on the growth & biochemistry of Brassica juncea and it’s impact on soil health. Journal of Environmental Science, Toxicology and Food Technology 8:22–39.
- Curtis WR, Paul MH, Alden HE. 1991. Modeling linear and variable growth in phosphate limited suspension cultures of opium poppy. Biotechnology and Bioengineering 38:371–79.
- Dormatey R, Sun C, Ali K, Qin T, Xu D, Bi Z, Bai J. 2021. Influence of phosphite supply in the MS medium on root morphological characteristics, fresh biomass and enzymatic behavior in five genotypes of potato (Solanum tuberosum L.). Horticulturae 7:265.
- Erin NN, Junairiah, Manuhara YSWM. 2020. The effect of IBA and Ethephon on growth and saponin content of Talinum paniculatum Gaertn. adventitious Root In vitro. Eco. Env. & Cons. 26 (June Suppl. Issue) : pp. (S9-S13)
- George EF, Michael AH, De Klerk GJ (editors). 2007. Plant propagation by tissue culture volume 1. The background. Dordrecht (Netherlands): Springer.
- Guol B, Abasi BH, Zeb A, Xu LL, Weil YH. 2011. Thidiazuron: A multidimensional plant growth regulator. African Journal of Biotechnology 10(45):8984–9000.
- Isda MN, Fatonah S. 2014. Induksi Akar pada Eksplan Tunas Anggrek Grammatophyllum scriptum secara In Vitro pada Media MS dengan Penambahan NAA dan BAP [Root induction of Grammatophyllum scriptum from shoot explant in vitro culture in MS medium supplemented with NAA and BAP]. Jurnal Biologi Al-kauniyah 7(2).
- Khristyana L, Endang A, Marsusi. 2005. Pertumbuhan, Kadar Saponin, dan Nitrogen Jaringan Tanaman Daun Sendok (Plantago major L.) pada pemberian Asam Giberelat (GA3) [Growth, saponin level and nitrogen content of Plantago major leaf tissue in gibberellic acid (GA3) treatment]. Biofarmasi 3(1):11–15.
- Kim JH, Chang EJ, Hoon-II O. 2005. Saponin production in submerged adventitious root culture of Panax ginseng as affected by culture condition and elicitors. Asia Pacific Journal of Molecular Biology and Biotechnology 13(2):87–91.
- Kirkby EA, Mengel K. 1987. Principles of plant nutrition 4th edition. Switzerland: International Potash Institute.
- Komatsu M. 1982. Studies on the constituents of Talinum paniculatum Gaertner. Zasshi Yagukaku 5:499–502.
- Lawlor DW, Lemaire G, Gastal F. 2001. Nitrogen, plant growth and crop yield. In: Lea PJ, Gaudry JF, editors. Plant Nitrogen. Berlin, Heidelberg: Springer-Verlag.
- Mahadi I, Wulandari S, Trisnawati D. 2013. Pengaruh Pemberian NAA dan Kinetin terhadap Pertumbuhan Eksplan Buah Naga (Hylocereus costaricensis) Melalui Teknik Kultur Jaringan secara in vitro [The effect of NAA dan kinetin on growth of Hylocereus costaricensis in vitro culture technique]. Jurnal Biogenesis 9(2):14–20.
- Manuhara YSW. 2014. Capita selecta plant tissue culture. Surabaya: Airlangga University Press.
- Manuhara YSW, Kristanti AN, Utami ESW, Arif Y. 2015. Effect of sucrose and potassium nitrate on biomass and saponin content of Talinum paniculatum Gaertn. hairy root in balloon-type bubble bioreactor. Asian Pacific Journal of Tropical Biomedicine 5(12):1027–32.
- Murashige T, Folke S. 1962. Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15:473–97.
- Oláh R, Szegedi E, Ruthner S, Korbuly J. 2003. Thidiazuron-induced regeneration and genetic transformation of grapevine root stock varieties. Vitis 42(3):133–36.
- Palestine, A.S, 2008, Induksi Akar Pada Biakan Tanaman Pule Pandak (Rauvolfia serpentine L.) Secara Kultur Jaringan [Root induction of Rauvolfia serpentine L. in vitro culture], thesis, Faculty of Agriculture, Malang
- Panda AK, Mishra S, Bisaria VS. 1992. Alkaloid production by plant cell suspension cultures of Holarrhena antidysenterica effect of major nutrients. Biotechnology Bioengineering 39:1043–51.
- Pavlov A, Ilieva M, Ivan NM. 2000, Nutrient medium optimization for rosmarinic acid production by Lavandula vera MM cell suspension. Biotechnol Prog.16:668–70.
- Preece JE. 1995. Can nutrient salts partially substitute for plant regulators?. Plant Tissue Culture and Biotechnology 1(1):26–27.
- Taiz L, Zeiger E. 2003. Plant physiology 3rd ed. Sunderland: Sinauer Associates.
- Solim MH, Kristanti AN, Manuhara YSW. 2017. Influence of Explant Position on Growth of Talinum paniculatum Gaertn. Adventitious Root in Solid Medium and Enhance Production Biomass in Balloon Type Bubble Bioreactor. IOP Conf. Series: Earth and Environmental Science 58 (01203)
- Wahidah S. 2011. Pengaruh Hormon Kinetin Terhadap Pertumbuhan KalusRumput Laut Kappaphycus alvarezii Melalui Kultur In Vitro [The effect of kinetin on callus growth of Kappaphycus alvarezii in vitro culture]. Jurnal Vokasi. Rev. 7(2):192–97.
- Wattimena GA. 1988. Zat Pengatur Tumbuh Tanaman. Bogor: LaboratoriumKultur Jaringan Bioteknologi IPB [Plant Growth Regulators. Bogor: Plant Tissue Culture Biotechnology. Institute of Agriculture Bogor].
- Widiyani T. 2006. Efek antifertilitas ekstrak akar som Jawa (Talinum paniculatumGaertn.) pada mencit (Mus musculus L.) jantan [The effect of antifertility of Talinum paniculatum Gaertn. root extract in male mice (Mus musculus L.)]. Bul. Penel. Kesehatan 34(3):119–28.
- Wiedenhoeft AC. 2006. The green world plant nutrition. New York: Infobase Publishing.
- Yin S, Liang Y, Gao W, Wang J, Jing S, Zhang Y, Li H. 2013. Influence of medium salt strength and nitrogen source on biomass and metabolite accumulation in adventitious root cultures of Pseudostellariaheterophylla. Acta Physiologiae Plantarum 35(8):2623–28.
- Zhang YH, Gao WY, Wang J, Li XL, Xiao PG. 2011. Improvement of growth and periplocin yield of Periploca sepium adventitious root cultures by altering nitrogen sources supply. Chin Herb Med. 3:226–31.